The Brazilian Medical Devices market is growing significantly and is expected to reach USD 1.8 billion by 2023. Medical devices in Brazil are regulated by the National Health Surveillance Agency i.e., ANVISA (Agência Nacional de Vigilância Sanitária). Medical devices using functionalities such as Bluetooth, Wi-Fi, Radio Frequency (RF), and other wireless interface require ANATEL certification and homologation as a pre-requisite for ANVISA registration. ANATEL (Agência Nacional de Telecomunicações) is the National Telecommunications Agency responsible for regulating all the telecommunication products marketed in Brazil.
ANATEL Homologation refers to granting of approval by the ANVISA’s compliance with Brazilian regulations and ensuring that the medical device is safe to use. The requirements for Brazil ANATEL Certification are defined in Resolution 715/2019. ANATEL Homologation plays an important role in completing the registration process of telecommunication products with the ANVISA. Medical devices with wireless technology aid in monitoring patients remotely or transferring patient data from the medical device to another platform such as a cell phone.
Classification of Telecommunication Products
ANATEL classifies telecommunication products into three (03) categories such as Category I, II, and III.
- Category I includes end-user products such as mobile phones, satellite phones, VOIP phones, mobile phone batteries, mobile phone charging cables, etc.
- Category II includes products requiring radio frequency such as TV and radio antenna, receivers, and transmitters, Wi-Fi equipment, and RF automation devices.
- Category III includes products such as optical fiber cables, mobile network signal transmitters and cable connectors.
Medical devices using RF wireless technology such as wireless cardiac monitors, implanted cardiac pacemakers and defibrillators, as well as neuromuscular stimulators, Radio Frequency Identification (RFID) wireless systems, and Handheld TENS (Transcutaneous Electrical Nerve Stimulation) devices fall under Category II of Telecommunication products. Each category must comply with different requirements for ANATEL Homologation.
- The Category I products must go through laboratory testing at an INMETRO accredited laboratory followed by an annual re-evaluation for maintenance of certification. The re-evaluation also involves laboratory testing.
- The Category II devices, like Category I products, must undergo laboratory testing at an INMETRO accredited laboratory followed by a bi-annual re-evaluation for maintenance of certification. The re-evaluation, in contrast to category I products, involves document verification and does not require laboratory testing. This is applicable to the wireless medical devices.
- The Category III products must undergo laboratory testing for certification, but there will be no periodic re-evaluation.
Step by step Process of ANATEL Certification and Homologation
The conformity assessment and ANATEL approval of Telecommunication products involve various stakeholders, including entities such as medical device manufacturers and Brazilian Registration Holder (BRH), assessment agencies such as testing laboratories and Designated Certification Body (OCD) and authorities like ANATEL.
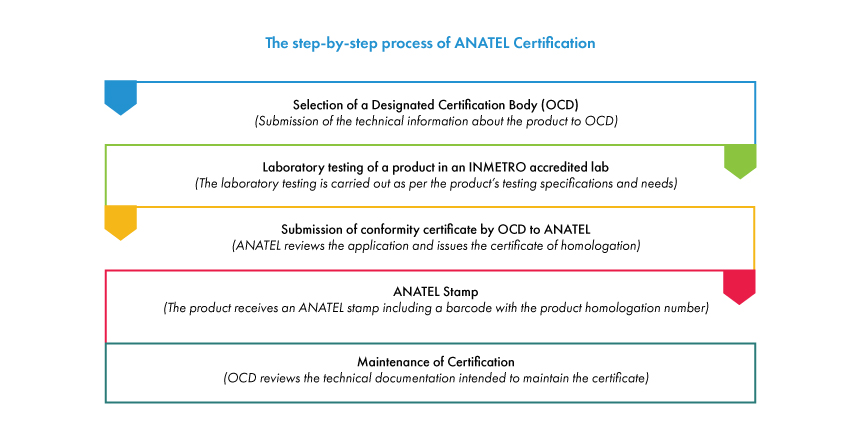
The duration for the entire ANATEL approval process is largely dependent on the type of the telecommunications product along with the time required for the completion of laboratory tests. ANATEL Brazil Certification is a mandatory requirement for the registration of telecommunication products with ANVISA. The ANATEL certificates do not have an expiry once issued by the OCD. However, the certificates are maintained periodically by the OCD for any change in the requirements of testing specifications.
For more information on the ANATEL certification and its process, kindly reach out to a regional Regulatory expert. Stay informed. Stay compliant.





