When the COVID-19 pandemic was first recognized as a global health crisis in 2020, global Health Authorities (HAs) took innovative steps to deal with it in the best possible manner. Likewise, pharmaceutical companies came up with several vaccines/medicinal products for the benefit of the public. However, registering the products turned out to be quite challenging. To simplify the registration while upkeeping their safety, quality, and efficacy, HAs granted Emergency Use Authorizations (EUAs) to a few vaccines and relevant products.
The question may arise as to how the HAs were able to ensure that the benefits of the said products outweighed the risks. The answer is the adoption of the best pharmacovigilance (PV) practices.
What is Pharmacovigilance?
PV is defined as a set of scientific activities related to detecting, assessing, understanding, and preventing adverse effects and any other drug-related problems. Following is a figurative explanation of how PV works and the process therein.
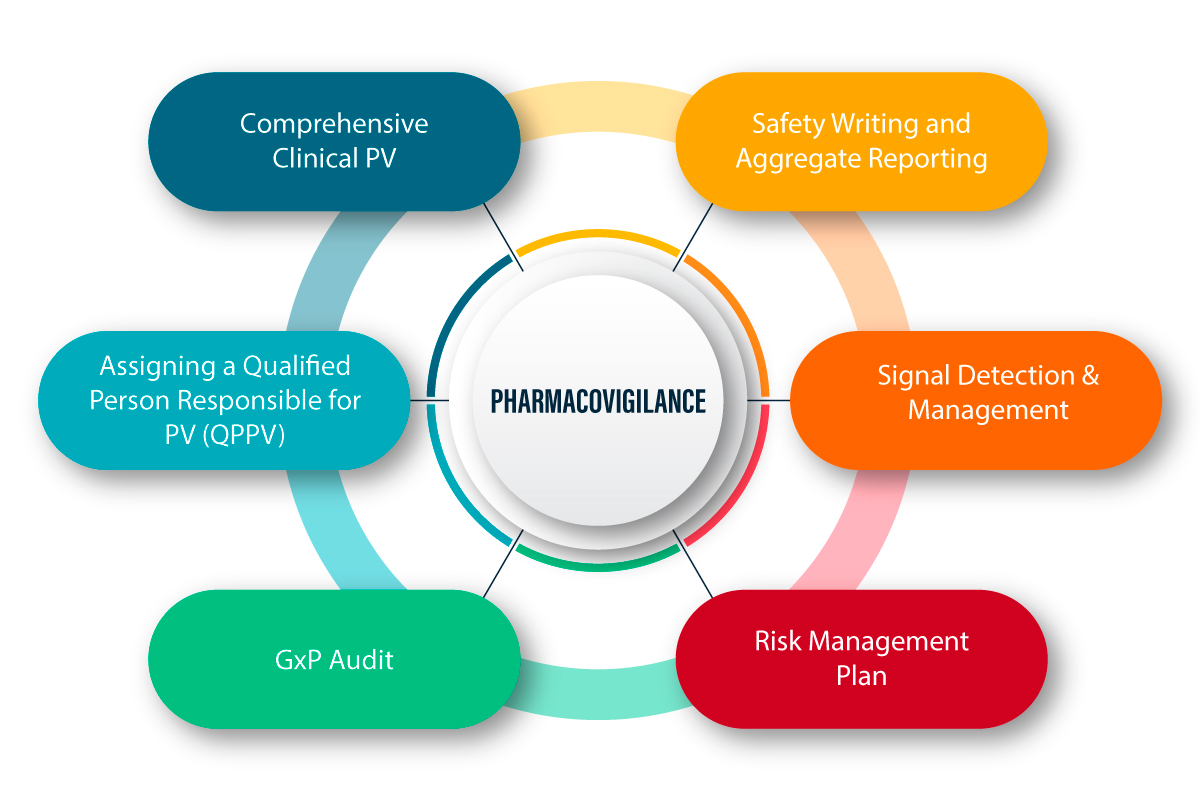
PV in the COVID-19 Pandemic
When the COVID-19 pandemic began to spread across the globe, vaccines and relevant medicinal products had to be introduced into the market in a limited period while maintaining the required quality standards. In such scenarios, there existed fewer subjects available for clinical trials during the development stage of drugs/vaccines, and the process had to be completed faster than normal. It could lead to certain adverse effects emerging in the post-approval phase.
Therefore, the benefit-risk balance of vaccines/medicinal products was considered paramount. With timely communication of adverse reactions and harmful side effects, the efficacy of the products was maintained on a real-time basis.
Some of the key PV activities that helped in maintaining the safety, quality, and efficacy of the said products in the pandemic are as follows:
- Adopting a good risk management plan
- Risk assessment with the help of Periodic Safety Update Reports (PSURs)
- Collation of the exposure data
- Post-authorization Safety Studies (PASS)
- Spontaneous reporting of suspected adverse reactions
- Effective signal management
PV in Regulatory Affairs (RA)
It is well known that the Regulatory Affairs team of a pharma company is responsible for the new products’ safety and approval. RA specialists take care of pharmacovigilance activities which proved to be crucial during the pandemic, such as:
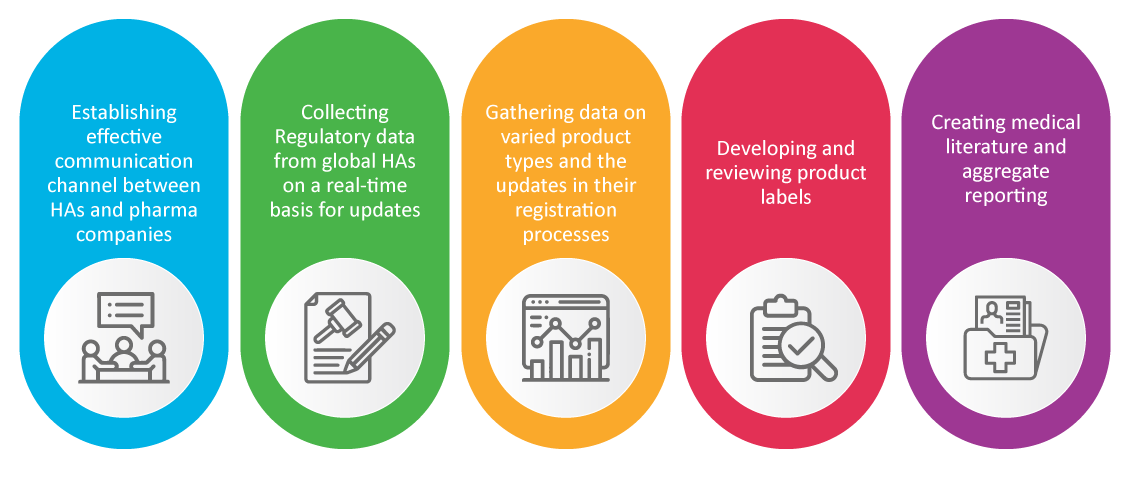
Conclusion
To conclude, PV helps monitor and report a drug product's safety. With customized Individual Case Study Reports (ICSR), it becomes easier for manufacturers/sponsors to comply with the respective regulations of global HAs. Are you looking for Regulatory support for ICSR preparation? Consult a proven Regulatory expert for compliance. Reach out to Freyr.





