Learn how AI is impacting Regulatory Affairs for Global Life Sciences Industry

Regulatory Affairs is evolving from a compliance function to a strategic business enabler. AI is the key to making that leap.
What This Whitepaper Explores:
- How AI and Gen AI are transforming regulatory operations
- Real-world AI use cases—from labeling automation to health authority Q&A prediction
- Why regulatory affairs is evolving from a compliance unit to a strategic business enabler
- ROI insights: Up to 50% faster submissions, 40% greater efficiency, and 2–5x ROI in 2–3 years
- Challenges and the path forward: Governance, integration, and the human-in-the-loop model
Fill the form below to download the White Paper
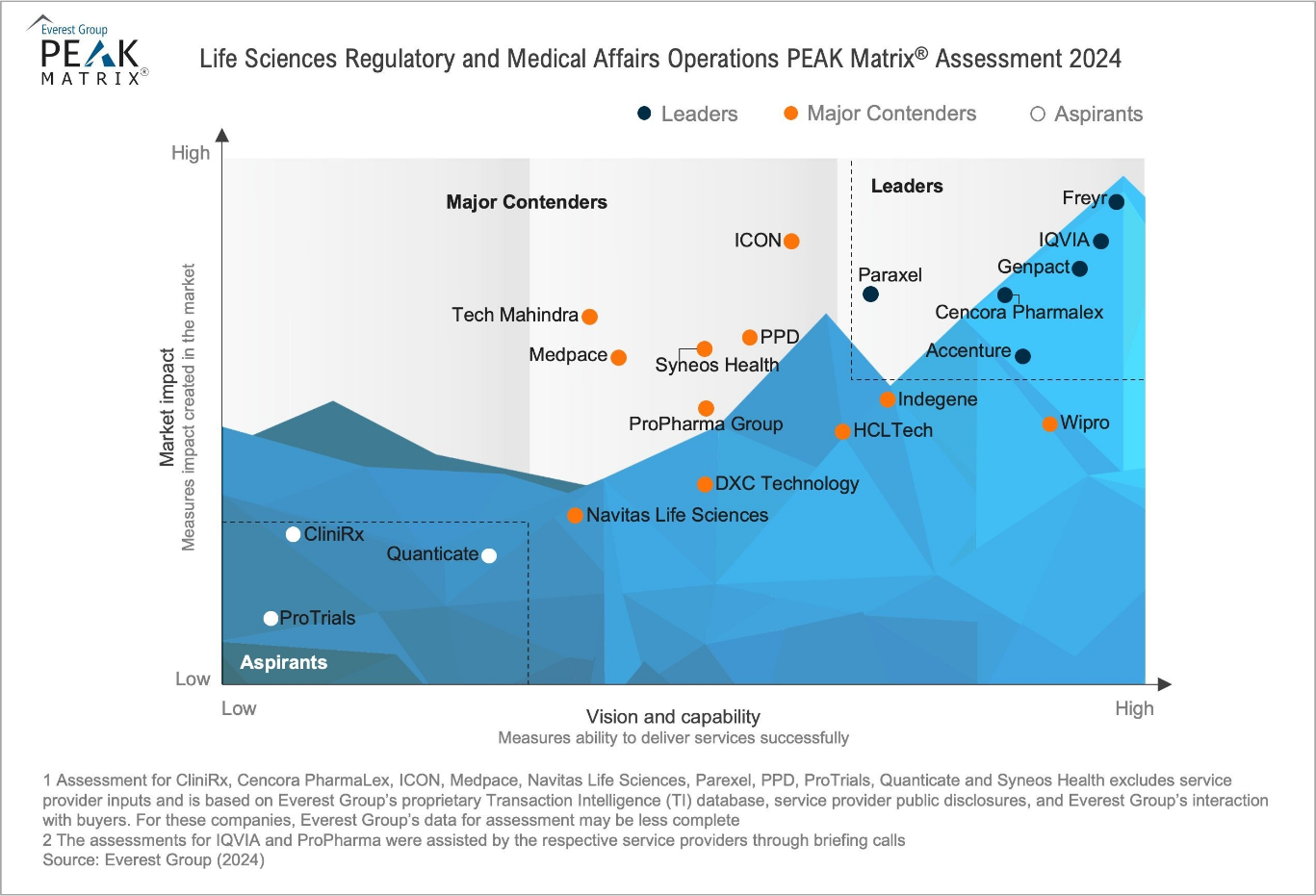
Why Regulatory Leaders choose Freyr?
Freyr has been recognized by Everest Group as a Leader in Life Sciences Regulatory and Medical Affairs Operations—making us a trusted Global partner for Life Sciences Industries.
Powered by innovations like Freya Fusion, Freyr’s AI-driven regulatory model, we help accelerate automation, streamline submissions, and enable faster approvals worldwide.
Let’s Transform Compliance into Competitive Advantage. Ready?
With 20+ global hubs, 400+ Regulatory Affairs Experts, and Partnerships with 200+ pharma companies, Freyr is the preferred Regulatory Partner for leading Life Sciences brands Worldwide.
The Future is AI- Driven. Lead the change with Freyr.
![Canada]() Schedule a
Schedule a
30-minute Consultation
![Canada]() Download Your
Download Your
Whitepaper
![Canada]() Get
Get
FREE LIVE DEMO
Look who is future ready with us.
 Download Your
Download Your





