The Largest Global
Regulatory Solutions and Services Provider
Our Story
2011
Commenced operations in the United States (US) with a small set-up and a 5-member core team.
Launched Freyr EVMPD.
Roped in our first customer, a Germany-based multinational pharmaceutical company.
![]()
2012
Ventured into Regulatory Affairs (RA) consulting.
Assisted an American multinational corporation with RA activities.
The team grew to 7 members.
![]()
2013
Set up a global operational center in Hyderabad, India.
Obtained International Organization for Standardization (ISO) certifications.
![]()
2014
Carried out major facility expansion in India.
Expanded the Regulatory team to 50+ members.
Launched Freyr IDENTITY – a Unique Device Identification (UDI) solution.
![]()
2015
Expanded footprints to the United Kingdom (UK).
The team grew to 100+ members.
Initiated Regulatory services for the Medical Devices segment.
Launched a specialized Centre of Excellence (CoE) for Medical Writing.
![]()
2016
Set up a Regulatory hub in Germany.
Received the “India Knowledge Process Services for Life Sciences Growth Excellence Award”.
Ranked as one of the finalists for “Excellence in Pharma: Regulatory Procedures and Compliance”.
The team size expanded to a total of 150+ experts.
![]()
2017
Crossed the 100+ customers milestone.
Established regional offices in Mexico and the United Arab Emirates (UAE).
Launched Freyr X and partnered with in-country affiliates across the globe.
![]()
2018
Flourished into a new phase of growth, with 250+ global customers.
Launched the new version of Freyr SUBMIT PRO – Freyr’s in-house electronic Common Technical Document (eCTD) tool.
Pushed geographical boundaries with new office spaces in Canada, Slovenia, and Sri Lanka.
![]()
2019
Developed Freyr LABEL 360 – an exclusive Regulatory labeling platform.
Expanded geographically, with a new office space in the US; also established a Regulatory hub in France.
Customer base increased to 450+.
Became a strong Regulatory team with 650+ members.
![]()
2020
Celebrated 100+ medical device approvals.
Launched the cosmetic brand, Slova.
Developed Freyr iREADY – an exclusive ingredient database platform for Cosmetics.
With the onset of COVID-19, prioritized the well-being of employees and customers with absolute remote operations.
![]()
2021
Supported 2 of the 3 major COVID-19 vaccine players.
Launched Freyr Digital to provide industry-leading digital transformation solutions using Artificial Intelligence (AI), Machine Learning (ML), and Robotic Process Automation (RPA) technologies.
Celebrated 200+ medical device approvals.
![]()
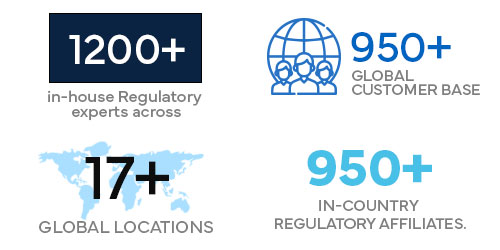
2022
1200+ in-house Regulatory experts across 17+ global locations.
950+ global customer base.
950+ in-country Regulatory affiliates.
![]()
2023
Opened new offices in Colombia and Poland.
Became a strong Regulatory team with 2300+ members.
1400+ global products supported worldwide.
1450+ global customers.
Launched Freya.
Won 2+ global awards.
Collected 1000+ kgs of groceries for donations.
![2023]()
![]()
Our Vision
To be the essential partner for managing global Regulatory complexities and market safe and compliant products across countries.
![]()
Our Mission
To pioneer Regulatory excellence through innovative solutions and expert guidance, empowering organizations to navigate compliance complexities seamlessly and drive transformative growth.
“Market has always been the guru for us. It is guiding us and shaping us at every step of our growth.”
Serving 120+ countries globally & counting..
End to end Regulatory services
Freyr provides comprehensive regulatory services, covering a wide range of 3,600 regulatory requirements. Our expertise spans strategy, submissions, and lifecycle maintenance for medicinal products, medical devices, cosmetics, food supplements, and chemicals. From inception to market and beyond, we are your trusted partner in navigating the complex regulatory landscape.
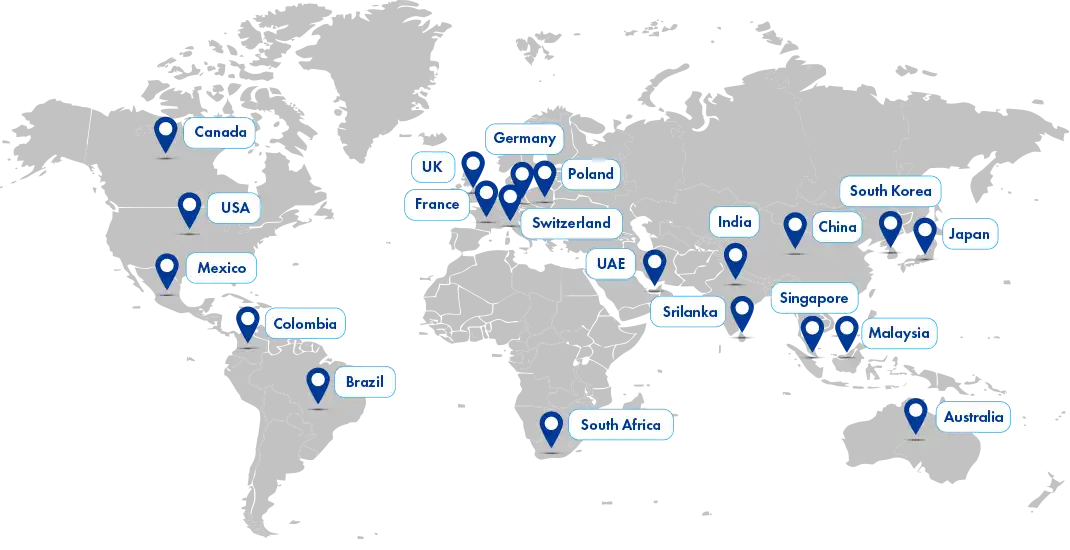
Our reach
We are dedicated to envisioning and ensuring global sustainability for local businesses while extending our reach to large organizations. With a strong presence in over 25 global locations and a vast regulatory affiliate network spanning 120+ countries, Freyr is well-equipped to support your regulatory needs worldwide.
Innovative approach
At Freyr, we empower organizations to enhance their compliance efforts with our AI-driven platforms and AI-integrated services. By embedding AI-enabled automation, we've elevated the quality, productivity, and timeliness of our customer deliveries, setting a new standard of excellence.