Summary of Product Characteristics (SmPC) is a medical monograph created and updated by pharmaceutical companies based on product research and knowledge. It offers much more than a structured list of vital information on each drug, such as fixed dosage and potential side effects. Pharmacists, doctors, nurses, and other healthcare professionals rely on this data to appropriately prescribe medications and educate their patients about medicinal product use.
SmPCs are reviewed and approved by the UK or European medicines licensing body, the UK Medicines and Healthcare Products Regulatory Agency (MHRA), and the European Medicines Agency (EMA). SmPCs are used by various healthcare providers, including pharmacists, to prescribe and use medication appropriately and safely. As new efficacy or safety data emerges, they are kept up-to-date throughout the medicinal product’s lifecycle.
The SmPC information is crucial for the correct and safe use and distribution of medicine. The SmPC consists of qualitative and quantitative information about each drug's benefits and hazards. The following information is required:
- The medication's composition
- Interactions with other medications
- Concomitant disease
- Organ impairment
- Effects on pediatric, geriatric, and pregnant populations
- Genetic variables and more
This life-or-death material is complete with instructions on what to do in emergency scenarios. That is why SmPC updates and accuracy are critical for patient safety.
What is an Abbreviated SmPC?
Abbreviated SmPC is a shorter/crisper version of the SmPC used to prepare promotional material in Europe. The abbreviated SmPC is usually four (04)-five (05) pages, and its template could vary from company to company, and it helps physicians with a quick review instead of the SmPC data. Only the very important data is presented in the abbreviated SmPC, unlike the SmPC. It is critical to maintaining the consistency of claims and the overall meaning of SmPC or abbreviated SmPC.
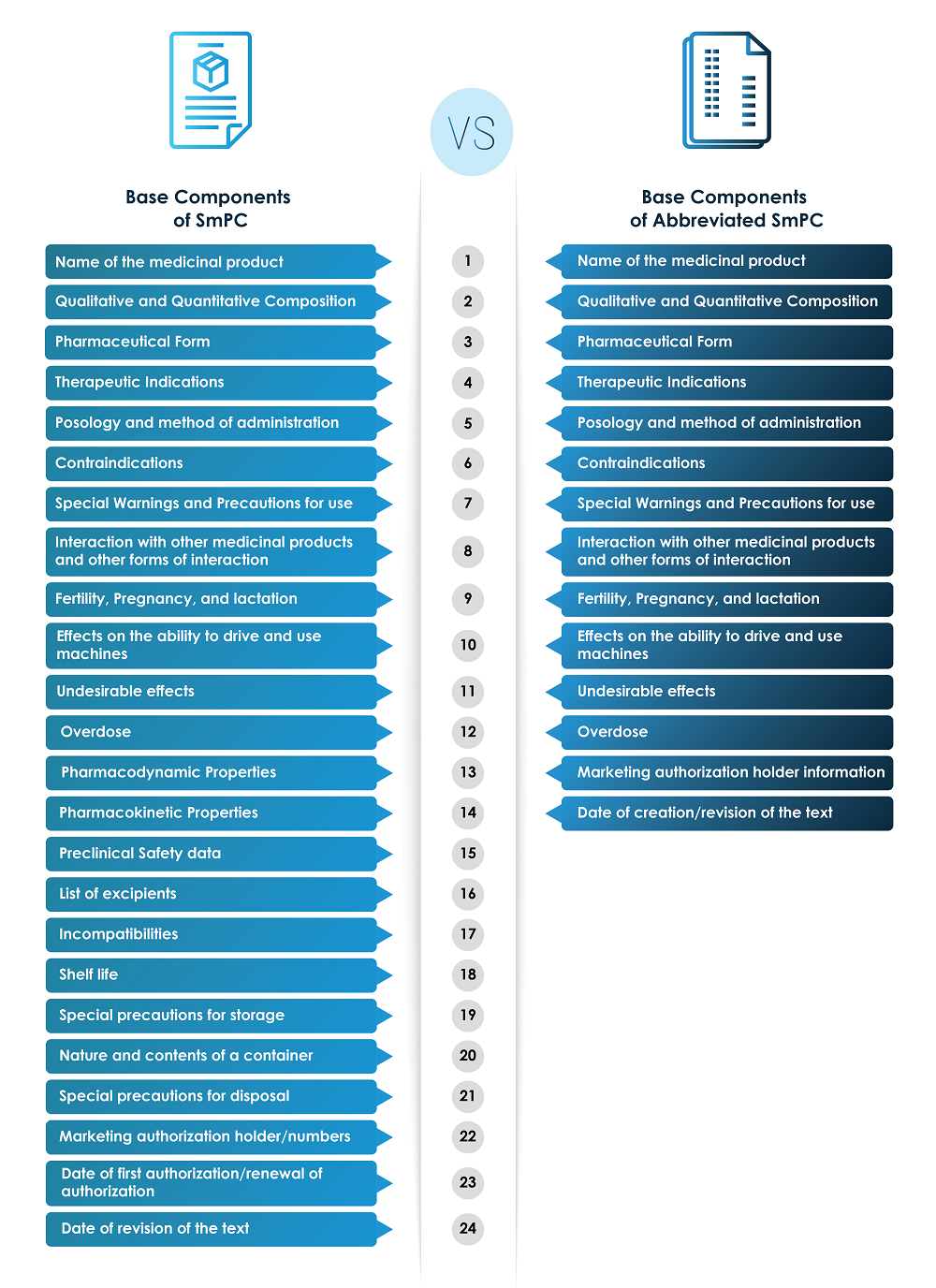
Abbreviated SmPC should contain an important note/mandatory statement at the top of the document, quoting, “Before prescribing, consult/refer to the full prescribing information.” The nomenclature could vary between Reduced PI, Basic Succinct Statement (BSS) document, and Abbreviated SmPCs from company to company. It also helps in defending legal cases arising due to promotional material. The following picture represents the components of both the SmPC and the Abbreviated SmPC.
Information on SmPC vs. Abbreviated SmPC
The Abbreviated SmPC preparation process varies from company to company. The source document which is used to prepare the Abbreviated SmPC must be an approved document, core label, or the latest approved SmPC. Post the 2nd draft, the translations are done if required before the medical or technical review. The comments are addressed, and then the final draft is rolled out. The below diagram illustrates the process of abbreviated SmPC preparation.
Abbreviated SmPC Preparation Process Flow
Various conditions can arise that necessitate a change to the SmPC or abbreviated SmPC, resulting in tens of thousands of revisions per year. Adverse drug events and changes in safety communication standards are the two most typical reasons for SmPC/ abbreviated SmPC upgrades. Adverse drug events that occur after a product has been released to the market are reported to national Health Authorities, and the responsible manufacturer is advised that an update is required.
Safety communication updates are regularly conducted to ensure compliance and patient safety criteria are met. SmPCs and abbreviated SmPCs are updated as compliance criteria change. Because this data is regularly updated during the medicinal product’s lifecycle, it can be challenging to track and manage, not to mention time-consuming. As the data represented in the abbreviated SmPC is in a crispier form, the time required to prepare an abbreviated SmPC is shorter as compared to the SmPC.
Our team of experts at Freyr have experience with products registered under multiple registration types and are experienced with creating and updating the Abbreviated SmPCs. Get in touch with us now!