Overview
With deep expertise in global Cosmetic regulatory landscapes, Freyr offers comprehensive Cosmetic product formulation services and Cosmetic ingredient review assessments to help brands stay compliant while creating safe, high-quality products. From Cosmetic ingredient analysis to formulation reviews and regulatory consultation, our team acts as your trusted Cosmetic formulation consultant, guiding you through the complexities of Cosmetic regulatory compliance across global markets.

Cosmetic Product Formulation and Cosmetic Ingredient Review
Every ingredient in a Cosmetic product plays a critical role in defining its formulation, efficacy, and claims. Even minor changes in ingredient type or concentration can significantly impact product performance and regulatory compliance. As such, countries worldwide have set stringent rules and restrictions on Cosmetic ingredients and their allowable limits.

The EU Cosmetic regulation bans over 1,300 ingredients and places limits on many others via Annexes II–VI of Regulation (EC) No 1223/2009.

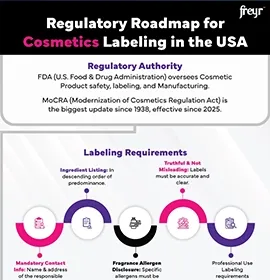
The US FDA restricts certain chemicals and color additives, with reviews conducted under the Cosmetic Ingredient Review (CIR) program.

ASEAN markets follow the ASEAN Cosmetic Directive (ACD), while Canada enforces its “Hotlist” of prohibited and restricted substances.
Given the periodic updates by Health Authorities (HAs), manufacturers must proactively monitor and comply with evolving ingredient restrictions to avoid non-compliance. Achieving this requires thorough Cosmetic safety assessments of all raw materials and finished formulations, in line with regulations such as EU 1223/2009, US FDA 21 CFR, Canada Hotlist, and now the US MoCRA compliance requirements.
Selecting the right ingredients and designing compliant formulations can be challenging. Freyr’s Cosmetic formulation services and Cosmetic product development support help brands overcome these challenges through robust ingredient reviews, toxicology assessments, and global compliance expertise.
How Can Freyr Help?
Why Choose Freyr?

Qualified experts with hands-on experience across all Cosmetic categories: skincare, haircare, baby care, oral care, and more.
Dedicated Cosmetic formulation consultant team providing origin-specific insights for ingredient acceptability.
Proven experience with over 2,500 Cosmetic ingredient review projects across synthetic, botanical, and mineral-based ingredients.
Regulatory change assessment support to ensure ongoing Cosmetic regulatory compliance.
End-to-end global regulatory consultation tailored to each region’s complexities.

Strong global HA relationships and a wide partner network to accelerate market access.

A structured approach ensuring quick and compliant product launches.
Cost-effective, hassle-free, and timely service delivery.
We understand that Cosmetic product development demands a perfect balance between innovation, safety, and compliance. With Freyr’s Cosmetic formulation services and regulatory expertise, brands can confidently bring safe and compliant products to the market, faster and more efficiently.
Frequently Asked Questions
We are here to provide you with the information you need quickly and efficiently
01. What is a Cosmetic ingredient review?
A Cosmetic ingredient review is a detailed assessment of each raw material and ingredient in a formulation to ensure it meets safety, efficacy, and regulatory standards. This includes checking against regional bans, limits, and permitted usage lists under EU cosmetic regulation, US FDA, Canada Hotlist, ASEAN ACD, and other guidelines.
02. Why is Cosmetic product formulation compliance critical?
Cosmetic product formulation must comply with global and country-specific regulations to ensure consumer safety and avoid penalties, recalls, or market rejections. Regulatory authorities periodically update permissible ingredients and limits, making ongoing Cosmetic regulatory compliance essential.
03. How does Freyr support Cosmetic product development?
Freyr provides end-to-end Cosmetic product development support through expert Cosmetic formulation services, Cosmetic ingredient analysis, Cosmetic toxicology reviews, and Cosmetic safety assessment, helping brands select the right ingredients and design formulations that meet both performance and compliance requirements.
04. What is MoCRA compliance, and why is it important?
MoCRA compliance refers to meeting the new Modernization of Cosmetics Regulation Act in the United States, which strengthens oversight of Cosmetic products, including mandatory ingredient safety substantiation and facility registration. Freyr helps brands adapt formulations and documentation to stay compliant under MoCRA.
05. How does a Cosmetic formulation consultant help?
A Cosmetic formulation consultant provides strategic guidance on selecting compliant ingredients, reviewing formulations against global regulations, optimizing for intended claims, and ensuring that the product is safe, effective, and legally marketable in your target regions.
06. What is included in Freyr’s Cosmetic formulation review services?
Freyr’s Cosmetic formulation review services include evaluating Cosmetic ingredient compliance, verifying concentration limits, assessing toxicological safety, checking for prohibited or restricted substances, and ensuring formulations meet the standards set by global regulatory bodies.
07. Does Freyr offer region-specific Cosmetic ingredient review assessments?
Yes. Freyr specializes in Cosmetic ingredient review assessments tailored to specific markets, such as EU Cosmetic regulation Annex II–VI, US FDA CIR, Canada Hotlist, ASEAN ACD, and others, helping brands achieve seamless compliance and quick market access.