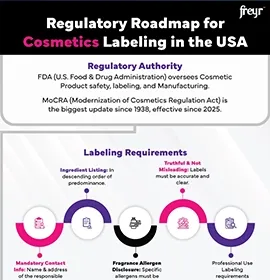
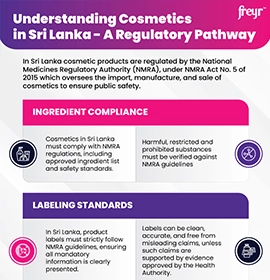
Overview
In today’s competitive beauty and personal care market, Cosmetic product claims play a critical role in influencing consumer perception and shaping brand identity. However, every Cosmetic claim, whether stated on packaging, labeling, digital platforms, or advertisements must be compliant with global and regional regulations. Non-compliant or misleading claims can lead to regulatory warnings, product recalls, enforcement actions, or delays in market entry.

What Is Cosmetic Claims Review?
A Cosmetic claims review is a detailed assessment of all statements that describe the benefits, and intended use of a Cosmetic product. These Cosmetic claims contributes to determine whether a product qualifies as a Cosmetic or crosses into therapeutic territory. Clear, compliant, and substantiated claims support marketability while ensuring alignment with regulatory standards worldwide.
A structured Cosmetic product claims validation can help you ensure that your Cosmetic claims are:
Truthful and non-misleading
Supported by evidence and accepted scientific rationale
Consistent with Cosmetic labeling and advertising compliance requirements
Appropriate for the intended Cosmetic product category
Compliant with market-specific cosmetic legislation and advertising standards across the US, EU, UK, ASEAN, and additional global regulations.
How Can Freyr Help?
Our review also helps identify Cosmetic claims that require stronger substantiation and ensures proper differentiation between cosmetic, borderline, and therapeutic claims.
Why Choose Freyr?

End-to-end global regulatory support

Cost-effective and scalable service delivery

Hassle-free, structured, and timely outputs

Strong relationship with global Health Authorities (HAs) and extensive partner network across the globe.

Strong understanding of global Cosmetic compliance services and country-specific nuances

A scientific and regulatory approach designed for faster market access

Global support with Cosmetic claims substantiation and claims consultation.

Qualified regulatory professionals with expertise across skin care, hair care, baby care, oral care, and other beauty categories
Ensure Your Cosmetic Claims Are Compliant, Credible, and Market-Ready
Freyr supports Cosmetic brands in creating compliant, compelling, and scientifically validated claims that resonate with consumers while meeting global regulatory expectations.
Looking for expert support in Cosmetic claims review and substantiation?
FAQs
1. What is a Cosmetic claims review?
A Cosmetic claims review is a structured assessment of all statements used on packaging, labels, websites, social media, to ensure the claims are truthful, non-misleading, substantiated, and compliant with regional regulations. It also checks that the claims remain within the cosmetic category.
2. Why do cosmetic claims need substantiation?
Regulators expect claims to be truthful, honest, and supported by credible evidence. Substantiation protects brands from warnings, recalls, enforcement actions, and market-entry delays, while enhancing consumer trust.
3. What types of claims are reviewed? Global support with Cosmetic claims substantiation and claims consultation. other
We review efficacy claims (e.g., “reduces frizz”), functional claims (e.g., “moisturizing”), ingredient-linked claims (e.g., “with Vit C”), safety/usage statements (e.g., “dermatologist tested”), any symbols (e.g., halal mark) and marketing language across labels, artworks, digital content, and ads.
4. How do you differentiate cosmetic vs. therapeutic claims?
Cosmetic claims describe cleaning, perfuming, changing appearance, protecting, keeping in good condition. Therapeutic claims imply treating, healing, correcting, or altering physiological functions. We review and recommend compliant alternatives to keep products within the cosmetic category.
5. What happens if a claim is not compliant?
If a claim is not compliant, it must be changed or supported with proper evidence before use. Otherwise, it can lead to regulatory warnings, delays, or product recalls. At Freyr, we help by rewriting the claim and recommending substantiation options to make it compliant.
6. Which markets’ regulations do you cover?
We provide global regulatory support for 120+ countries. Reviews are tailored to country-specific nuances, labeling rules, and advertising standards, ensuring claims remain valid across all target markets.
7. How does Freyr help beyond review?
Freyr provides end to end consultation from claims strategy and copy optimization to scientific substantiation, label/artwork alignment, language translation services and global compliance guidance. We also support borderline analysis and ongoing updates as regulations evolve.
8. Can we use “dermatologist tested,” “hypoallergenic,” or “clinically proven”?
Yes, if supported by evidence. These phrases carry higher substantiation thresholds. We assess your study design/data and advise on safe wording that meets regional expectations (US, EU, UK, ASEAN, etc.).