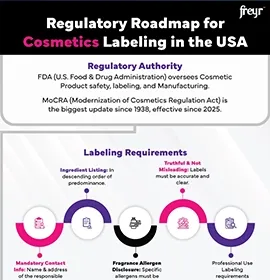
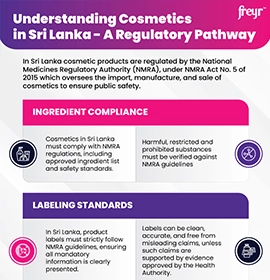
Overview
Entering international cosmetic markets requires strict adherence to region-specific Regulatory frameworks, and non-compliance can lead to product rejections, customs delays, fines, or complete market withdrawal. Appointing a Cosmetics Legal Representative or a Cosmetics Responsible Person is not just a regulatory formality, it is a mandatory requirement in many regions such as the EU, UK, LATAM and Asian countries.

What is Go-To-Market and Cosmetic Legal Representative (LR) Services
Go-To-Market Services and Cosmetic Legal Representative (LR) services are specialized regulatory support functions designed to help cosmetic brands enter global markets smoothly and compliantly.
A Cosmetic Legal Representative (LR), also known as a Cosmetics Responsible Person in markets like the EU and UK, Asian and LATAM is the officially designated entity responsible for ensuring that your cosmetic products meet all applicable Regulatory requirements in a specific country or region. This includes product safety oversight, documentation management, Dossier/product information file (PIF) maintenance, and acting as a formal point of contact for Health Authorities.
On the other hand, Go-To-Market Services provide end-to-end regulatory and strategic support to help manufacturers, distributors, and brand owners prepare their cosmetic products for launch across global markets. These services encompass product classification, formulation review, labeling and claims assessment, dossier preparation, PIF review and compilation , regulatory strategy, and managing mandatory submissions such as Cosmetic Product Notification or regional Cosmetics registration
Your cosmetic products meet safety, quality, labeling, and claims requirements.
All required technical and regulatory documentation is compliant and submission ready.
Health Authority interactions and mandatory notifications are managed professionally.
Your product is fully prepared for market-entry, reducing delays, risks, and compliance gaps.
How Can Freyr Help?
Why Choose Freyr?

Complete, end-to-end cosmetic Regulatory consultation

Experienced regulatory professionals across all cosmetic product categories

Region-specific expertise to navigate global regulatory complexities

Strong global partner network supporting seamless submissions

Established relationships with global Health Authorities

Structured, cost-effective approaches enabling quicker market access
Make Your Cosmetics Market-Ready and Compliant
Freyr ensures smooth global launches with Go-To-Market and Legal Representation Services, covering product safety, labeling, documentation, and regulatory approvals.
Need expert support for Cosmetic Legal Representation and compliance?
FAQs
1. What is a Cosmetic Legal Representative (LR) or Responsible Person (RP)?
A Cosmetic Legal Representative (LR) also called a Responsible Person (RP) in regions like the EU and UK is the officially designated entity accountable for ensuring your cosmetic products comply with all local regulations.
2. What do Cosmetic Legal Representative (LR) or Responsible person (RP) do?
Cosmetic Legal Representative (LR) or the Responsible Person (RP) oversee product safety, maintain documentation (including the Product Information File (PIF)/ Dossier), manage notification or registration, and serve as the formal point of contact for Health Authorities.
3. Do I need an LR/RP to sell cosmetics internationally?
In many regions, yes, it's mandatory. The EU and UK require a designated RP for every cosmetic product placed on the market. Asian and LATAM markets also mandate LR or equivalent compliance oversight. Without an LR/RP, your products may face customs delays, fines, refusals, or market withdrawal.
4. What do Go-To-Market services include for cosmetics?
These services cover end-to-end regulatory & launch readiness including product classification, formulation review, ingredient compliance, labeling & claims assessment, regulatory strategy, Dossier & PIF review & compilation & handling mandatory submissions like Cosmetic Notification & Registration.
5. What are the risks of launching without proper compliance or LR/RP?
The risks of launching without proper compliance or LR/RP include:
- Product rejection or seizure at customs
- Fines & regulatory penalties
- Forced recalls or market withdrawal
- Reputational damage & business delays
Appointing an LR and following a robust Go-To-Market plan helps avoid these outcomes.
6. How does Freyr ensure ongoing compliance after launch?
We provide regulatory monitoring, update labels/claims as rules evolve, maintain the PIF/Technical File, support audits, & manage Health Authority interactions. We also issue regulatory intelligence alerts & strategic roadmaps to keep your products aligned with changing requirements across markets.
7. What’s the difference between a Legal Representative and a Responsible Person?
They’re often the same role. In the EU and UK, the LR is formally called the Responsible Person (RP), in Asian & LATAM countries we generally use the term LR, while in some countries like Japan and South Korea the term MAH also can be used.