The Conformity Assessment Body (CAB) carries out the conformity assessment of various stakeholders of medical device supply chain system. The Conformity Assessment Body (CAB), Malaysia carries out extensive audits and evaluates all the applicable elements set by the Medical Device Authority (MDA), Malaysia and verifies the device conformity to the relevant standards.
This part of the blog series will take you through the role of CABs in device registration and approval in Malaysia. You can also gain insight on the MDA's requirement for CAB certification, which has been covered in the first part.
Conformity assessment is a systematic and ongoing examination of evidence and procedures to ensure the safety, performance, benefit and risk of medical devices. It also ensures the manufacturing compliance to Essential Principles of Safety and Performance (EPSP) and requirements of the Medical Device Act 2012 (Act 737). The classification of a medical device determines the conformity assessment procedures to be undertaken. Conformity assessment becomes more stringent as the risk of medical device increases; hence the medical device risk assessment is necessary.
The CABs carry out various types of assessments depending on the business operations carried out by the organization and the application in scope. They include:
- Assessment of technical documentation
- Assessment of QMS systems for ISO 13485 compliance
- Good Distribution Practice for Medical Devices (GDPMD)certification
- Full conformity assessment for device registration
- Simplified verification for device registration
The CABs play a critical role for each stakeholder in the supply chain system including device manufacturers, importers, distributors, Authorized Representative (AR) and others.
The importers, distributors and Authorized Representatives require their establishments to be registered with the MDA to carry out their operations. The GDPMD certification is an essential pre-requisite, to be submitted along with the application for establishment license. The CABs carry out the conformity assessment of the Quality Systems of these entities for compliance with the GDPMD regulations set by the MDA before issuing the certificate. This assessment includes review of technical documentation as well as the onsite audit of the facilities.
The device manufacturers are also dependent on the CABs to get their device registered with the MDA, Malaysia. The domestic manufacturers require their manufacturing facilities to be registered with the MDA. the CAB.
In addition to the establishment registration, the CABs also play their part in device registration with the MDA. Medical devices with reference country approvals are subjected to the CAB verification, whereas the devices with no reference country approvals shall undergo full assessment by the CAB.
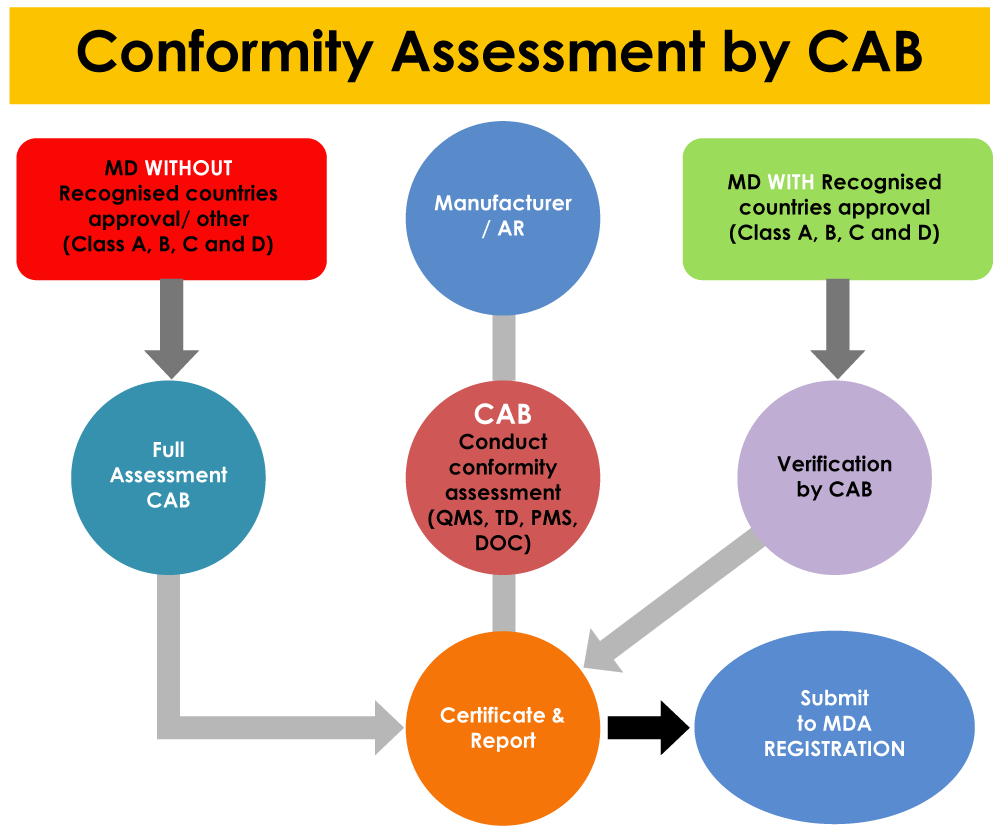
If there are any non-conformities found in the QMS, PMS system and complete device dossier (including each and every test report), there is a high possibility for the application to be rejected by the CAB.
Read part 1 of the article to know the MDA’s requirements and approval procedures for CAB certification.
Are you aiming to launch your medical device or IVD in the Malaysian market? Would you like to gain further insights on the CAB roles and responsibilities for device registration? Reach out to a Regulatory expert. Stay informed. Stay compliant.









