In addition to completing the requisite xEVMPD data submissions, life sciences organizations will now need to use a web-based application form called the Digital Application Dataset Integration (DADI). The European Medicines Agency (EMA) designed DADI as a long-term replacement for the PDF-based electronic Application Form (eAF), which was incepted to support eCTD submissions.
The timelines for this transition have been released recently by the EMA. DADI will replace the form for variations of medicinal products in the year 2022. Other submission forms for Centrally Authorized Products (CAP) and Nationally Authorized Products (NAP) will soon be followed by 2023. The project will also replace forms used for the key EU procedures, including the Centralized Procedure (CP), Mutual Recognition Procedure (MRP), Decentralised Procedure (DCP), and National Procedure (NP).
The web forms (inclusive of Human Variations Form (HVF) for medicinal products meant for humans) will make the inputs uniform for eAFs to provide standard product master data for medicinal products. They will enable both the existing human-readable and the new machine-readable output for digital processing, based on the Fast Healthcare Interoperability Resources (FHIR) data exchange standard for medicinal products. They will also use existing product master data from Product Management Services (PMS) to prepopulate the form fields wherever applicable.
The DADI web-based forms are intended to enable more methodical processing of an application, therefore reducing the organization’s workload. For example, errors and discrepancies will be reduced because of the forms supporting scrutiny of submitted applications by Health Authorities. The forms will also clear the way for “first-time-right” data being fed into databases, thus making integration of systems and sharing of data between Health Authorities effortless. Furthermore, the new forms will replace the archaic technology.
Benefits of DADI
The aim of DADI and this data-driven transformation of Regulatory processes can be summarized as follows:
- To enhance efficiency across Regulatory, R&D, and manufacturing functions
- To communicate with Health Authorities in a faster way
- To enable data-driven decision-making for both firms and Authorities
- To have better insights as well as foresight of business outcomes and overall performance
Holistically, this aims to be beneficial for patients, regulators, as well as the industry.
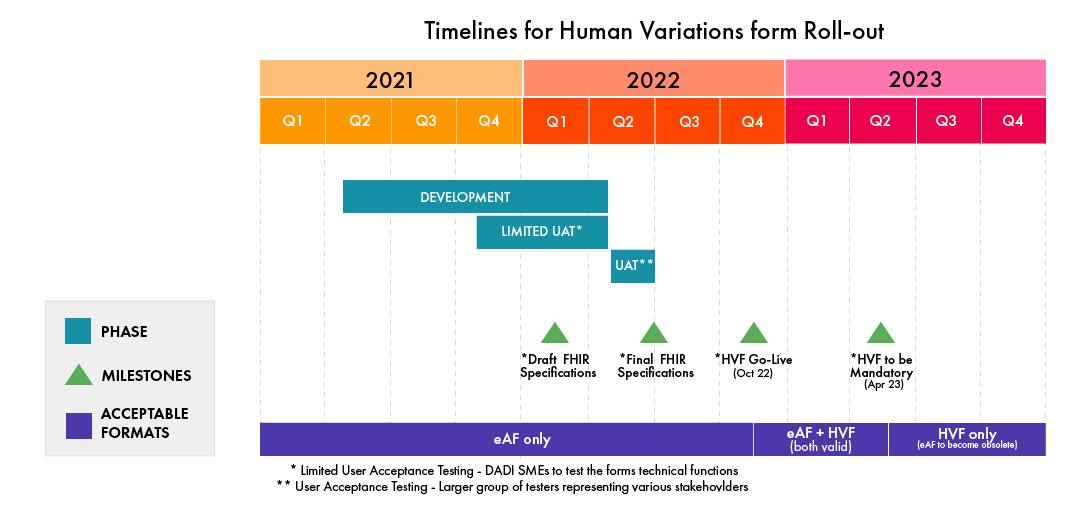
Latest Timeline Changes by the EMA
- The go-live date for HVFs has been pushed to October 2022 from the earlier decided timeline of April 2022.
- Presently, the HVF is undergoing closed User Acceptance Testing (UAT) by a group consisting of Subject Matter Experts on DADI.
- Subsequently, an integrated UAT will take place with a larger group of various stakeholders in Q2 of 2022.
- Once it goes live, there will be a six (06)-month transition phase in which both the eAFs (old PDF version) and the web-based forms will be accepted parallelly. Post this, only the HVF will be accepted.
Please refer to the following infographic to note the timelines for the HVF implementation (Updated in February 2022, subject to changes by the EMA):
(source: EMA)
Ensuring Adaptability to the Dynamic Regulatory Environment
All major Health Authorities are now moving towards the submission of high-quality data sets and documents. It is important for organizations to maintain the same in a unified system.
It is also crucial to have a clear understanding of the entire submission lifecycle - its requirements, the people and processes associated with it, and the current site of source data by a thorough need assessment. Data cleansing and enrichment must be taken on priority if the assessment indicates a need.
It is imperative to have a Regulatory Information Management System (RIMS) in place that adapts to evolving regulations and guidelines and is also flexible to reshape the organization’s current needs and complies with the dynamic Regulatory environment. To know more about Freyr’s suite of Regulatory solutions, which will make your organization ready for DADI and more such process alterations, visit us at Freyr Digital.