After the initial discovery of nitrosamine impurities in drugs and Active Pharmaceutical Ingredients (APIs) by the USFDA in mid-2018, the EU Regulatory bodies have also joined many other countries in a bid to prevent the risks involved. They have re-called several medicines that post health perils from the substance. Known for its carcinogenic properties, Nitrosamine may prove to be harmful when ingested above the accepted levels by humans. The substance, N-nitrosodimethylamine (NDMA), has an acceptable limit of 96 ng/day and anything above this is objectionable in drugs and APIs.
Nitrosamines are formed when secondary, tertiary, or quaternary amines react with a nitrosating agent. All the drugs that have chemically synthesized active ingredients are being checked for impurities. The Committee for Medicinal Products for Human Use (CHMP) has reviewed and created an assessment report. It has asked Marketing Authorization Holders (MAHs) to follow the latest guidance for reviewing all the chemical and biological medicines currently available in the market for human consumption.
MAHs are responsible for making sure that the manufacturing process of all the biological and chemical products is reviewed periodically to identify any contamination. Once identified, the relevant steps must be taken to allay the risks they pose. Though the chances of such contamination are less during the manufacturing of chemical and biological medicines drugs, the European Medicines Agency (EMA) is not taking any chances there. Pharmaceutical companies need to ensure that the relevant manufacturing protocols are in place as per the latest EMA guidelines. They are also responsible for checking the levels of nitrosamine in drugs that are available in the market and keeping them within the acceptable limit.
Post finalizing a review under Article 5(3) of Regulation (EC) No 726/2004 (call for review) last year; the EMA has issued new guidance for the avoidance of the presence of nitrosamine contamination in human medicines. The process is the same for nationally authorized and centrally authorized products. The EMA, along with the European Directorate for the Quality of Medicines & HealthCare (EDQM), will be implementing the CHMP Article 5(3). Here is the process that should be followed to remain compliant with the modifications.
The three-step guidance for MAHs
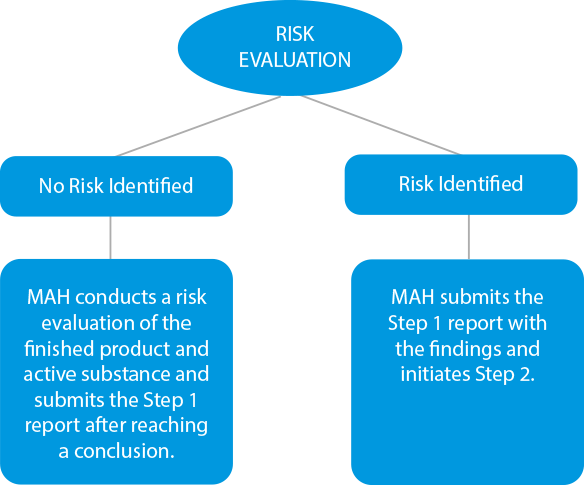
- Risk Evaluation - Manufacturers need to conduct a risk evaluation process to identify the active substances and the finished product to check the nitrosamine levels. If there are any instances of cross-contamination, the same should also be included in the outcome report. The deadlines for submissions at this stage were set for March 31, 2021, for chemical medicines, and July 01, 2021, for biological drugs.
![]()
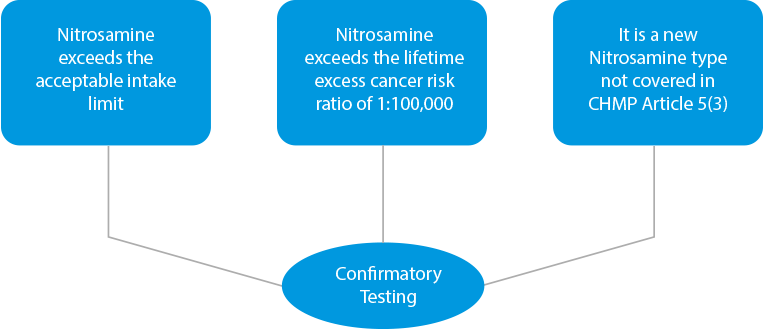
- Confirmatory Testing – In case of any cross-contamination findings or if products are identified as risky due to the presence of higher levels of Nitrosamine, confirmatory testing needs to be performed. Confirmatory testing is mandatory in three (03) instances.
![]()
- Marketing Authorization Modifications – When the presence of nitrosamine is detected, two (02) confirmatory tests need to be performed to report the correct readings to the EMA. Based on these, the MAHs must apply for changing the manufacturing process. This is done by using the standard Regulatory procedures with the help of a variation to the Marketing Authorization Template. The timelines for these are September 26, 2022, for chemical products, and July 01, 2023, for biological medicines.
Ideal Way Forward for MAHs to Deal with the New Nitrosamine Level Compliance
Since this is the latest development that the EMA has proposed to mitigate the risks involved with Nitrosamine levels in drugs and a cross-contamination situation, the whole process can be quite overwhelming for MAHs and API manufacturers. Whether it is submitting the Response Template when contamination is detected at Step 1 or conducting consequent testing, every phase has to be compliant with the new rules. MAHs need to collaborate with Regulatory experts who are up to date with all the latest changes and ensure compliance with the new guidance. Choose the right partner like Freyr to avoid any delays and errors.