
Effective January 31, 2022, the new European Union (EU) pharmaceutical regulation for Clinical Trials Regulation (CTR) has become mandatory, repealing the Clinical Trials Directive 2001/20/EC. The regulation harmonizes protocols for evaluating and supervising clinical trials across the EU. The guidelines were revised to promote a uniform approach to clinical research while stressing clinical trial participants’ safety and increased public disclosure.
The regulation establishes a new two-part evaluation system for all EU clinical trials. Part I consists of a scientific evaluation of the core clinical trial paperwork, and Part II consists of an ethical evaluation of documentation at the national level. Following this two-part evaluation, each Member State will make a unified decision on the trial and notify the sponsor via the Clinical Trials Information System.
Transition Timelines for New Applicants
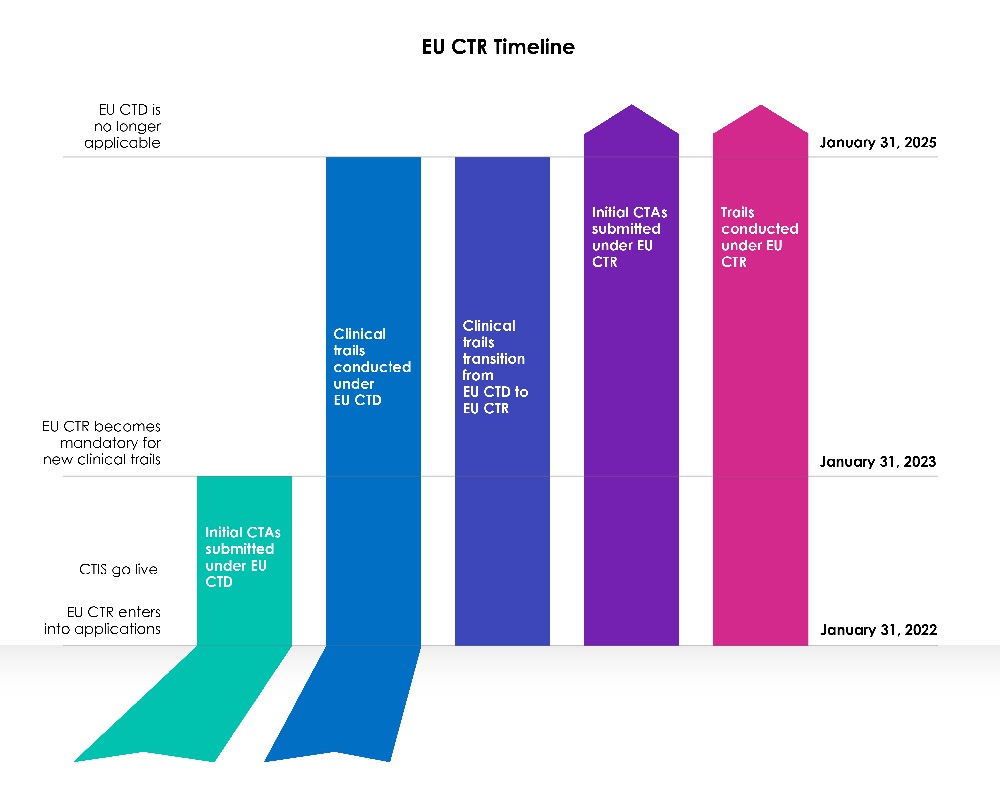
A three (03) year transition phase began with the EU CTIS go-live date.
Year 1 (January 31, 2022, to January 30, 2023):
The European Union (EU) Clinical Trial Directive 2001/20/EC (EU-CTD) has governed clinical trials in the EU since 2004. It strived to standardize rules and significantly improved patient safety in clinical trials. However, in practice, it created unintended consequences. During the first year, following the CTIS implementation, sponsors were allowed to choose whether to apply for a new Clinical Trial Application (CTA) under the Clinical Trial Information System (CTIS) under the Clinical Trial Directive (CTD: Directive 2001/20/EC), or to use CTIS in accordance with the current legislation, the Clinical Trial Regulation (EU) No 536/2014.
Both ideas were viable, and sponsors got a chance to pick which legislation to pursue.
Members were ready to use the Clinical Trial Information System (CTIS) and accepted applications under the new legislation, Clinical Trials Regulation (EU CTR), on the first day of CTIS operation.
Years 2 and 3 (January 31, 2023, to January 31, 2025):
Beginning January 31, 2023, all new CT Applications must be submitted via the CTIS under the new legislation (CTR).
New CT Applications are not allowed to be submitted in EudraCT under the Clinical Trial Directive (CTD). The EU Clinical Trial Directive no longer allows for the admission of new Member States after January 31, 2023. Trials conducted under the CTD must first be transitioned, after which an additional Member State-Concerned application can be submitted via EU CTIS.
For Existing Applicants
CT Applications submitted before January 30, 2023, under the old legislation (CTD) using EudraCT, will be permitted to run until the completion under that Directive ((CTD: Directive 2001/20/EC), until January 30, 2025. Procedures will remain unchanged, and sponsors will be able to submit significant modifications and end-of-trial notices as required by the regulation. EudraCT will remain active during the transition time to allow these trials to continue.
It is, however, important to note that transition applications can be submitted at any time during the three (03) year transition period, and sponsors are encouraged to complete the process early enough in the transition period to ensure the EU clinical trial continuity beyond January 30, 2025, considering statutory holidays and the two (02) week winter clock stop.
Non-transferable Trials
- Trials that have either currently concluded or will conclude just before the end of the EU/EEA transition period must not be transitioned.
- If an end-of-trial notification has been completed in all EU/EEA member countries, but the global end of the trial hasn’t yet been notified, the study should not be transitioned. Under the Directive, the global end of the trial and trial summary results should be posted via EudraCT.
- Trials that began prior to the implementation of Directive 2001/20/EC do not benefit from such a transition procedure. If they are interventional and must continue to operate after the CTR transition phase ends, a new CT Application must be issued under the CTR.
- Pediatric trials conducted outside the EU/EEA but for which a EudraCT number has been assigned should not be converted as well.
- Trials that are on hold after the transition period has ended cannot be transitioned. Restarting the trial in these circumstances would necessitate the CT Application submission of a fresh application under the CTR.
EU CTIS receives technical updates from the EMA regularly to improve its features and functionality. When major changes are made to the CTIS, the EMA issues release notes outlining what has changed in the system. Updates may include enhancements to existing features and functionality, the addition of new features, and functional & technical enhancements. A seasoned Regulatory partner can address potential challenges and help sponsors with the trial transition for existing and prospective trials as part of clinical development strategies. Click here to learn more about the CTIS and Freyr’s expertise in the area: https://regulatoryaffairs.freyrsolutions.com/clinical-trial-applications-ctas.









