International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is an international non-profit organization that strives to maintain an updated database to summon Regulatory Agencies and pharmaceutical manufacturers to discuss scientific and technical aspects of the industry on a single platform. Medical Dictionary for Regulatory Activities (MedDRA), owned and developed by ICH, is a distinct and standardized Regulatory dictionary that facilitates the communication of Regulatory information across the globe, seamlessly. Similarly, pioneering in clinical terminology, Systematized Nomenclature of Medicine (SNOMED) International is a not-for-profit organization that maintains the world’s most comprehensive terminology database, Systematized Nomenclature of Medicine -- Clinical Terms (SNOMED CT), which includes over 350,000 concepts ranging across diagnosis, signs, and symptoms.
In a study published in 2009, the feasibility of using SNOMED CT as an entry point for coding adverse drug reactions and mapping them automatically to MedDRA for reporting purposes and interoperability with legacy repositories was analyzed. Understanding the scope of such a collaboration, ICH and SNOMED as a joint effort, announced the release of important new maps in Regulatory, and clinical space. Collaborative efforts under the project WEB-RADR 2, led to the release of two (02) important roadmaps (MedDRA to SNOMED CT and SNOMED CT to MedDRA) which have been structured around repeatability of term usage and additional key pharmacovigilance MedDRA terms identified by the European Medical Agency (EMA). To promote drug safety, interoperability among the Pharmacovigilance database (MedDRA) and Electronic health records (SNOMED CT) can help identify possible side effects and activate adverse events reporting simultaneously. The data collected through such reports can be useful for conducting epidemiological research in patient demography. Key elements associated with MedDRA adverse event reporting could be used to associate adverse drug events while providing “aid in clinical decision making”.
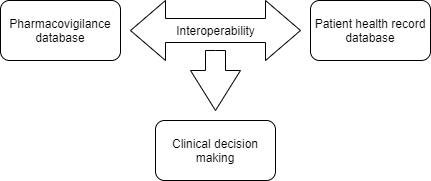
The production version of the two maps is being made available to licensed SNOMED CT and MedDRA users from April 30, 2021, onwards and will be based on the January 2021 version of SNOMED CT and the September 2020 version of MedDRA. It has been decided that the maps will be released annually in April.
To access the maps:
- Licensed MedDRA users, visit the Downloads page on the MedDRA website
- Licensed SNOMED CT users visit SNOMED International
Recent updates regarding, established interoperability between pharmacovigilance database and Patient health records may seem complex to navigate through. However, Freyr’s experienced Regulatory professionals can function as a single point of contact to provide technical support across patient health database and enhanced clinical decision-making needs. To enhance the quality output of your Pharmacovigilance requirements, we provide assistance across; ICSR, Aggregate reports, Qualified Person Responsible for PV (QPPV) Services, the US Agent services, Signal Detection & Evaluation, Database Migration, Adverse Event Reconciliation and Local Affiliate Services and much more. To explore Freyr’s end-to-end Pharmacovigilance capability, contact us now! Stay informed. Stay compliant.