
The need for life-saving drugs is on a constant rise. Owing to novel diseases proving fatal, drug manufacturers are spending a lot of time and money on the research and development of chemical and biological drugs. As challenging as inventing Innovator drugs is, it can be equally daunting for manufacturers to register them. From preparing and submitting the Investigational New Drug Application (IND) to Clinical Trial Applications (CTAs) and Marketing Authorizations (MAs), there are a series of Regulatory procedures to be followed, as prescribed by the respective Health Authorities (HAs).
Manufacturers must get it right the first time so that the Innovator drugs reach the market without delays. This will help meet the demand for life-saving drugs in specific markets and ensure Return on Investment (ROI) for the manufacturers.
The need for enhanced Regulatory solutions and compliant processes is, therefore, quite crucial. There are several reasons due to which manufacturers are unable to follow the appropriate pathway.
Regulatory Challenges Faced by the Innovator Drug Manufacturers
- Lack of understanding of the Regulatory complexities
- Limited knowledge of the various stages of submissions to be made to the HAs
- Timelines for submitting the medical dossiers, Common Technical Document (CTD), Clinical Study Reports (CSR), etc.
- Differences in regulations when the drugs are to be sold globally
- Lack of preparedness for dealing with any issues or difficulties arising in the manufacturing stage
Advantages of Collaborating with the Right Regulatory Services/Solutions Provider:
The right partner will ensure that all the drug-manufacturing steps are compliant with the relevant Regulatory bodies and lead to timely submissions.
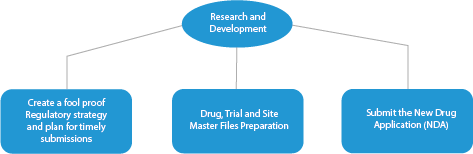
There are four (04) stages of Innovator Drug manufacture. These are as follows:
- Research and Development
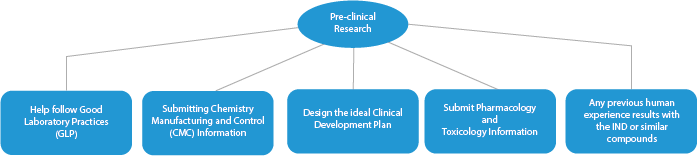
- Pre-clinical Research/Trials
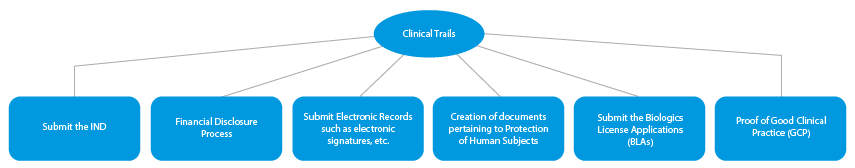
- Clinical Trials
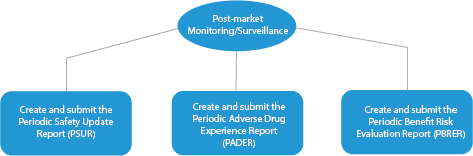
- Post-market Monitoring/Surveillance
Every stage of drug development requires a series of documents that need to be submitted to the HAs. Here is a depiction of how an established Regulatory services provider will help in all the phases.
Final Word
With the increasing life expectancy among the global population, there is a need to discover new drugs. Innovation in the life-sciences field has led to several inventions in life-saving drugs. Many of them are at various stages of manufacturing. Whatever the stage your product is in, collaborating with a global Regulatory service provider like Freyr, who has expertise in registering Innovator drugs, can help you avoid non-compliance and ensure that the new drugs reach the market on time. Stay informed. Stay compliant.









