Quality is paramount in the pharmaceutical industry. From research and development to manufacturing and distribution, every step must adhere to the highest standards of accuracy to ensure patient safety and Regulatory compliance. One of the major aspects of this intricate process is pharmaceutical labeling, specifically the artwork flow. This crucial element maintains product quality, prevents errors, and streamlines the pharmaceutical production cycle.
Optimize Your Labeling. Contact Us
Optimize Your Labeling Now!
Artwork Flow: A Critical Component
Artwork in pharmaceutical labeling refers to the visual elements, text, and graphics found on medication packaging, including labels, inserts, and boxes. These components contain vital information, such as dosage instructions, indications, contraindications, and safety warnings. Consequently, any errors or inconsistencies in the artwork can lead to patient harm, legal repercussions, and damage to a company's reputation.
Artwork flow encompasses the end-to-end process of creating, reviewing, approving, and managing these visual assets. It involves collaboration among various departments, such as Regulatory Affairs, quality control, marketing, and production. Augmenting this process is essential for ensuring the correct and updated information reaches the final packaging and labeling materials.
Here is a representation of the ideal artwork process flow that ensures labeling compliance:
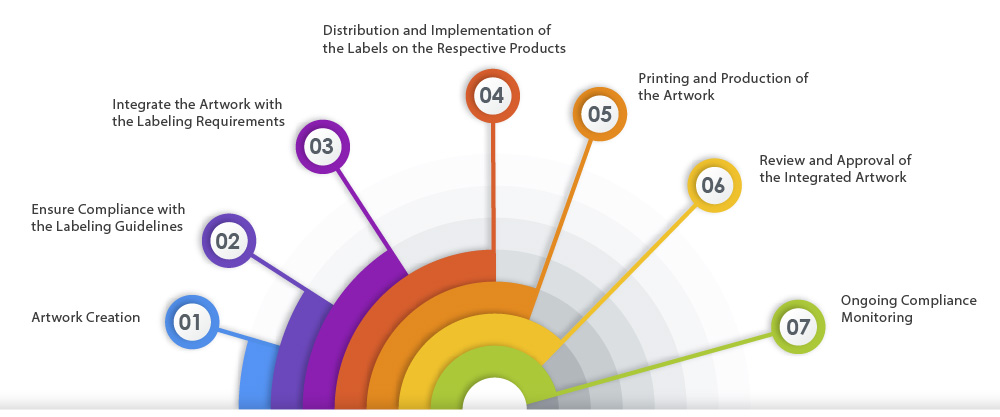
Challenges in Artwork Flow
The pharmaceutical industry faces unique challenges when it comes to managing artwork flow, such as the below:
- Regulatory Compliance: The pharmaceutical sector is highly regulated, with strict guidelines and requirements from Health Authorities such as the United States Food and Drug Administration (US FDA), the European Medicines Agency (EMA), and others. Artwork must adhere to these regulations, which can vary by region and product type.
- Complex Review Processes: Artwork must go through rigorous review processes involving multiple stakeholders. Coordinating the feedback and ensuring everyone is on the same page can be time-consuming and error-prone.
- Version Control: With numerous iterations and updates to artwork, keeping track of versions and changes is crucial. Mismanaged version control can lead to outdated or inaccurate information on packaging.
- Globalization: Many pharmaceutical companies operate globally, necessitating translations and adaptations of artwork for different languages, markets, and cultures.
- Time Pressure: The pharmaceutical industry often operates under tight timelines, especially during product launches. Rushing the artwork flow process increases the risk of errors.
Enhancing Artwork Flow
A robust artwork flow optimization strategy is essential to address these challenges and ensure efficient pharmaceutical labeling. This can be achieved by implementing the following:
- Centralized Digital Management: Implement a digital artwork management system that centralizes all artwork-related data, making it easily accessible to authorized stakeholders. Such a system ensures a single source of information and reduces the risk of using outdated versions.
- Automated Workflows: Establish automated workflows for artwork review and approval. These will reduce manual errors, accelerate the process, and maintain a transparent audit trail of changes.
- Collaborative Tools: Utilize collaboration tools that facilitate communication and feedback among different departments and teams involved in the artwork process.
- Regulatory Expertise: Employ experts who understand pharmaceutical labeling requirements. Their Regulatory insights will help ensure compliance with the ever-evolving regulations.
- Quality Control Measures: Implement quality checks and validation processes to identify errors early in the artwork creation.
- Change Management: Develop a change-management strategy to handle updates, revisions, and recalls effectively.
Conclusion
Accurate labeling ensures patient well-being and Regulatory adherence. The artwork flow streamlines the entire process and detects errors that could lead to risks. By optimizing the artwork flow strategy through the strategic use of technology, collaboration, and Regulatory expertise, pharmaceutical companies can safeguard their products and reputation and prioritize accurate and compliant labeling.
Partnering with a labeling expert can hasten Regulatory approvals and ensure compliance with global regulations. Consult Freyr to know more about our labeling competence.