
The development and approval of medications for children is a critical aspect of pediatric healthcare. However, due to ethical and practical considerations, clinical trials involving children are often limited. As a result, pediatric drug labeling plays a crucial role in ensuring the safe and appropriate use of medications in this vulnerable population. In this blog, we will compare the pediatric drug labeling requirements in the European Union (EU) and the United States (US) to gain a better understanding of the similarities and differences between these two (02) Regulatory frameworks.
EU Pediatric Labeling Requirements
The EU has specific requirements for pediatric labeling of medicinal products. These requirements ensure that medicines intended for children are appropriately tested, authorized, and labeled to provide safe and effective treatment options for pediatric patients.
Ensure Pediatric Drug Safety. Contact Us
Ensure Pediatric Drug Safety Now
Here are some aspects of EU pediatric labeling requirements:
- Pediatric Investigation Plans (PIPs): A PIP must be submitted to the European Medicines Agency (EMA) before a medicine can be approved for children. It outlines the studies and data required to assess the medicine's safety, efficacy, and dosing in pediatric populations.
- Pediatric Use Marketing Authorization (PUMA): If a medicine has been studied in children and meets the requirements, it may receive a PUMA. This authorization allows the medicine to be marketed for pediatric use.
- Age-appropriate Formulations: Medicines for pediatric use should be available in formulations suitable for different age groups, such as liquids, chewable tablets, or age-appropriate dosing devices.
- Pediatric-specific Summary of Product Characteristics (SmPC): The SmPC is a document that provides detailed information about a medicine. For pediatric products, it should include specific details on dosing, administration, and safety considerations for different age groups.
- Pediatric Warnings and Precautions: The labeling should include any specific warnings or precautions related to the use of the medicine in children, such as potential side effects or interactions with other medications commonly used in pediatric patients.
- Ongoing Monitoring and Updates: Once a medicine is approved for pediatric use, post-authorization studies may be required to gather additional data on its safety and efficacy. These can lead to updates in the labeling as necessary.
US Pediatric Labeling Requirements
In the US, the Food and Drug Administration (FDA) has implemented the Pediatric Research Equity Act (PREA) and the Best Pharmaceuticals for Children Act (BPCA) to promote pediatric drug research and labeling. PREA requires pharmaceutical companies to conduct pediatric studies for certain drugs likely to be used in children. The BPCA grants an additional six (06) months of market exclusivity to companies conducting these studies.
The FDA enforces further guidelines to ensure pediatric drug safety, efficacy, and proper dosing as follows:
- Pediatric Study Plans (PSPs): Manufacturers must submit plans outlining pediatric studies for drug safety and efficacy evaluation.
- Age-specific Information: Labels should include age-specific dosing, indications, and safety considerations.
- Weight-Based Dosing: Weight-dependent dosing recommendations ensure accurate administration.
- Adverse Reactions: Provide pediatric-specific adverse reactions and safety data.
- Pediatric-specific Formulations: Drugs may need pediatric-specific dosage forms (e.g., liquids and chewable tablets).
- Human Factor Engineering: Labels should consider ease of administration for caregivers.
- Patient Information Leaflets: Clear instructions for administration, dosing, and side effects are required.
The below table represents the differences and similarities between the US and EU labeling requirements:
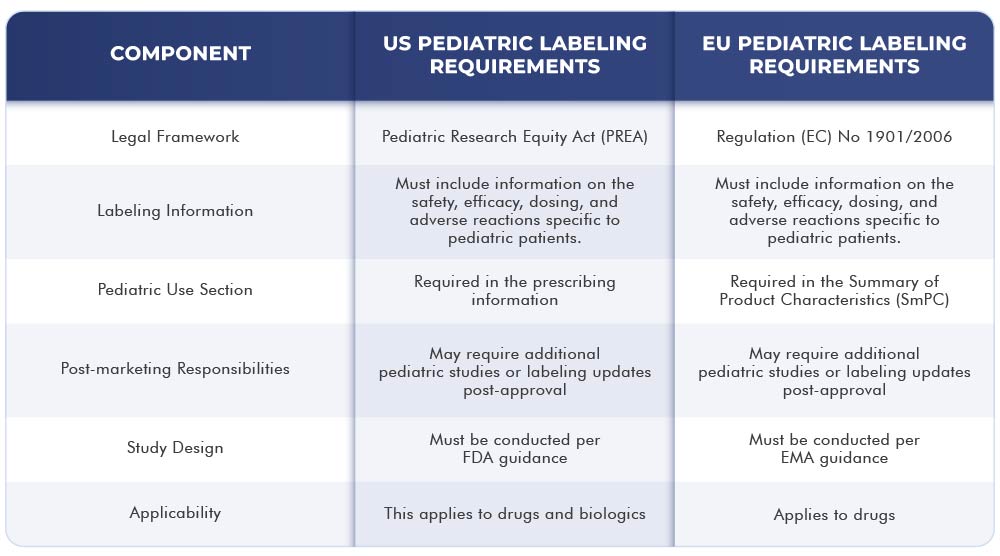
Conclusion
Pediatric drug labeling requirements in the EU and US aim to ensure the safe and appropriate use of medications in children. While there are similarities between the two Regulatory frameworks, such as the need for pediatric studies and the inclusion of pediatric-specific information in drug labeling, there are also notable differences. Understanding these similarities and differences is crucial for pharmaceutical companies, healthcare providers, and regulators to ensure children have access to safe and effective medications.
Consult a proven labeling expert like Freyr for compliance with country-specific labeling requirements.









