
Pharmaceutical advertising, including direct-to-consumer advertising, is regulated by the United States Food and Drug Administration’s (US FDA) Office of Prescription Drug Promotion (OPDP), Advertising and Promotional Labeling Branch (APLB) at the Center for Drug Evaluation and Research (CDER), and the Center for Biologics Evaluation and Research (CBER) respectively. The information submitted to the OPDP and the APLB must be accurate, ethical, and non-misleading. Additional information about the product’s benefits and risks must be included in the submission. The Offices also review promotional materials submitted to the Agency.
In April 2022, the US FDA published guidance on the submissions for promotional labeling and advertising material. The guidance helps in understanding the electronic submission in Module 1 of the eCTD, using version 3.3 or higher of the US-regional-backbone file. A mention of the types of promotional materials not subject to the mandatory electronic submission under section 745A is enlisted in the document. Paper copies of all the promotional submission types will be accepted until twenty-four (24) months after the guidance publication.
Exception
The document states that the submissions under section 505(b), (i), or (j) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) and submissions under section 47 351(a) or (k) of the Public Health Service (PHS) Act must be submitted in an electronic format as defined by the FDA. This document is not subject to the usual restrictions in the FDA’s good guidance practice regulations as it is not established legally for enforceable responsibilities. Therefore, the portion of this guidance that establishes the requirement for electronic submissions under section 745A(a) of the FD&C Act has a binding effect as indicated by the use of the words must, shall, or required.
The FDA reviews the drafted promotional material within forty-five (45) days of the voluntary submission by the sponsors. For any queries related to the product raised by the healthcare professionals, consumers, drug sponsors, or law firms, the OPDP grants the sponsors to acknowledge the same within thirty (30) calendar days.
Definition of Promotional Material
The expression ‘promotional material’ refers to promotional labeling and advertising materials regardless of the format, manner, or medium through which they are communicated. The FDA generally oversees two (02) types of labeling for drugs:
- FDA-required labeling
- Promotional labeling
As per the FDA’s section 201(m), labeling is defined as “all labels besides written, printed, or graphic embossed on any container, wrapper, or on any article or accompanying such an article.” The language used in ‘accompanying such an article’ is considered an interpretation or explanation of the promotional material, as stated by the US Supreme court.
Criteria for Submitting Promotional Material for Review
- Inclusion of suitable NDA, ANDA, or BLA numbers
- In cases where the applicants require immediate review, address submissions to the OPDP Project Manager
- From the form FDA 2253, allocate the most specific type of material to represent the promotional material
- Different types of materials are to be submitted separately
- Do not mix other submissions with promotional material
- Promotional material directed toward healthcare professionals must be submitted separately from the ones directed to consumers
The below metrics clarify the count of forms 2253 submitted and the materials included within these submissions.
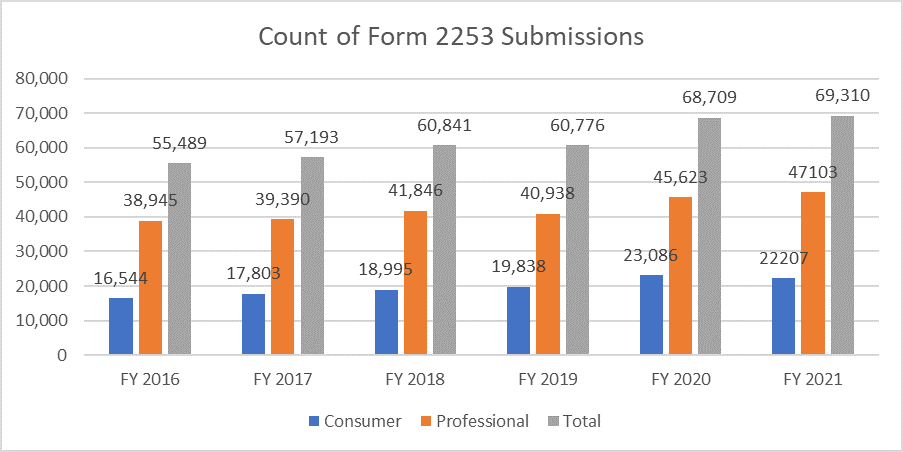
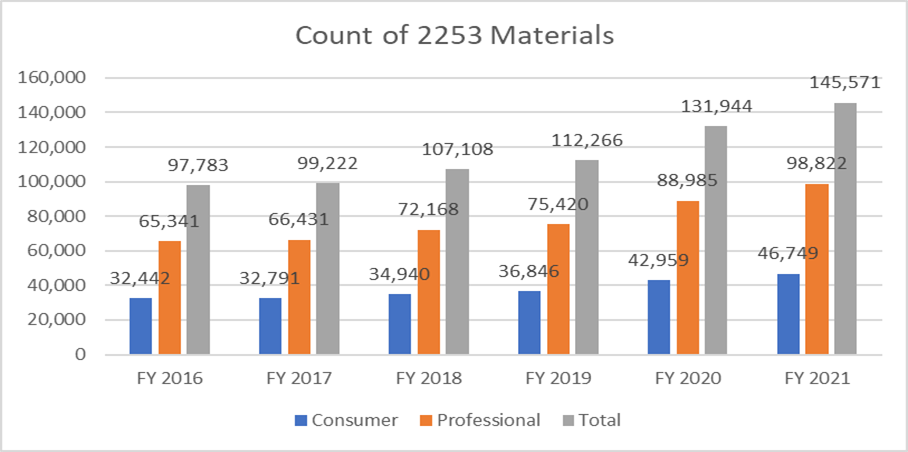
It is of utmost importance that the sponsors draft their promotional material as per the guidance provided by the FDA to avoid multiple review cycles, given the cost of the process. Having resources who can help facilitate the process of submitting promotional material seamlessly can enable the sponsors to achieve their business goals and stay at par with the Regulatory requirements. A proven Regulatory partner like Freyr can ensure a complete review of promotional and non-promotional material before the submission, whether in an electronic or non-electronic format. Contact Freyr today to draft clear, concise, and compliant promotional material right the first time.









