
Regulatory Affairs Staff augmentation is a trusted and proven staffing model that infuses the right talent at the right place at the right time into the life science organization’s flexible workforce plan.
Intended to provisionally enhance the internal team with expert resources cost-effectively and flexibly to meet the specific regulatory affairs staffing needs and solve the complex issues around urgent project deadlines and critical skillset shortages.
In this era of unprecedented market uncertainty, where we are witnessing hiring freezes and corporate layoffs, life sciences organizations need to be more flexible and agile than ever to adapt to the changing market dynamics and resource needs.
To that end, the evaluation of "flexible staffing models for talent management," as the range of staff composition varies according to specific needs becomes necessary. It must meet long-term and short-term staffing needs and provide a strategic framework for adding and removing staff in an efficient, timely, and cost-effective manner.
Staff augmentation has established its importance over the years, and there are numerous ways and benefits to achieve this.
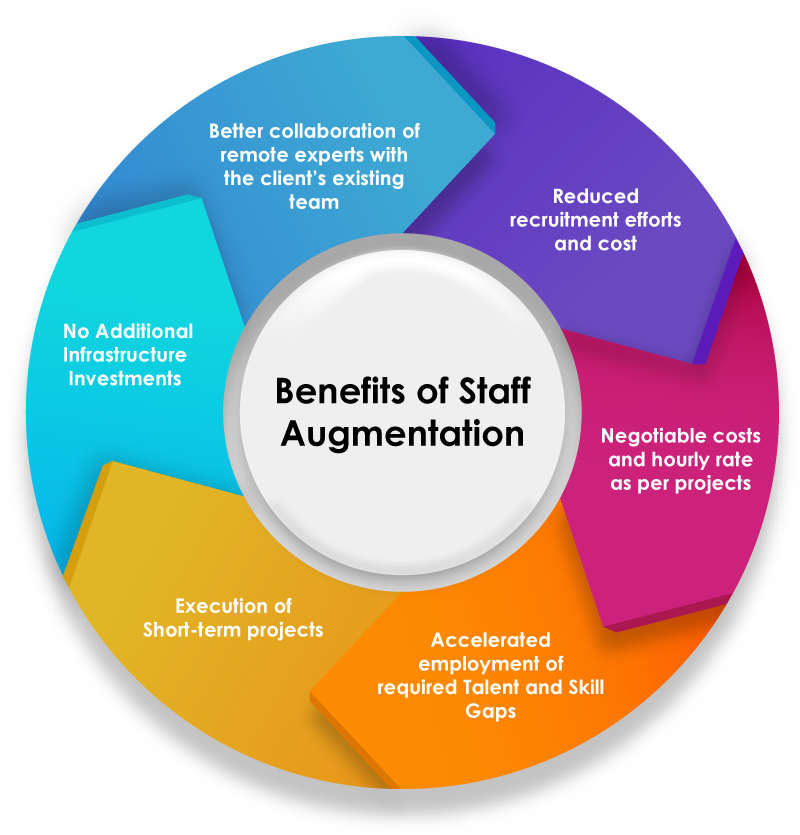
Benefits of Regulatory Affairs (RA) Staffing Outsourcing
RA Staffing Models for Life Sciences
Functional Service Provider (FSP) Model
In this type of staffing model, pharmaceutical companies are flexible in choosing the staff as per their requirement. If the company wants full-time staff for a project, it can choose to contract out staff for full-time till the work is done. However, if the company wants partial staff for a specific task, it can choose to contract partially. This model gives the organization flexibility to pick and choose which services to outsource to which FSP partner based on its expertise. Hence companies can either outsource the staff for all the RA-related tasks, such as submissions, drug safety, management, and HA monitoring, or a few tasks, such as validation and SOP writing.
Types of FSP models:
- Full-Time Equivalent (FTE) Staffing Model: This is a comprehensive analytical staffing strategy that allows pharmaceutical companies to have highly skilled staff with Regulatory expertise. In this model, the number of working hours required to complete a project is considered rather than the number of staff working. The experts through this model can provide their expertise across the globe as well as in different time zones. This approach of staffing is beneficial when there is a short, or mid-term need for staff due to time-bound projects.
- Fixed Price Model: In this model of staffing, the budget of the contract is agreed upon in advance, regardless of the time and expense. The organization knows the total cost to finish all the assigned tasks to the employees. This model also allows organizations to control costs and risks. Hence, the vendor is obliged to complete the contract.
| Pros | Cons |
| A single contract can support many projects for the company | Staff management burden on the organization as the expansion of the team requires oversight and management |
| Improves administrative transparency and reduces associated costs | There is a chance of process handoff issues if the various FSPs are involved in multiple RA requirements |
Full-Service Outsourcing (FSO) model
In this type of staffing model, pharmaceutical organizations usually outsource either majority of the research-oriented tasks or the whole research work to the RA experts. Contracts are more unit based rather than FTE based in the FSO. In this model, organizations outsource full responsibility to the staffing partners within the scope of the preferred relationships with the vendor. Hence organizations outsource the entire drug development process to a full-service provider.
Type of FSO model:
- Unit-Based Staffing Model: In this model, the organizations pay for units of work which is delivered by the staff. This model is used where the volume of work or tasks flexes up and down for a specific period. The hours per unit and volume of work are predictable, well-established, and understood. The resources are carefully measured and utilized in this model.
| Pros | Cons |
| Reduces management burden on the organization as the whole work is outsourced at once | Contracts per project may reduce efficiency, as the staff is sometimes not ready for add-ons |
| The workforce requires constant change, and the organization is flexible in bringing additional staff to address the business needs | No control of the organization over staff, as the staff is outsourced, and the internal manager might not be aware of employees' specialties |
Having a staff augmentation model in place facilitates flexibility, control, and cost-effectiveness in the system. Whether you are looking for Regulatory experts to help in your business or want to scale up the expertise of your existing RA team, we have global reach and Regulatory expertise to meet your demands to eliminate skill gaps and reinforce your projects. Contact Freyr.









