While innovators or branded drug products carry additional costs associated with the research and development of the product from the molecule identification stage to post-market authorization, generic drug products are relatively cost-effective. These therapies not only reduce the overall cost of treatment, but also significantly improve patient access to life-saving drug products. Since reliance on the import of generic antibiotics and rare-disease drug therapy affected the availability of these drug products globally amidst the pandemic, health agencies are introducing new guidelines to support local drug manufacturers.
Since 1993, the Medicines for Europe trade organization, formerly known as the European Generics Medicine Association (EGA), has been consistent with its objective to familiarize cost-effective therapy to the European patient population. Over the past decade, they have successfully supplied 67% of all medicines to Europe, amplifying patient access to 100% in seven (07) key therapeutic areas. Within the purview of the European health authorities, a “Strategic autonomy” post-Brexit set into motion an immediate requirement to effectively remedy the competitive disadvantage faced by the European generics and biosimilars manufacturers. With the advent of the COVID-19 pandemic, the global supply chain capabilities succumbed under the pressure of drug demands. Life-saving drugs that provided relief from chronic illnesses disappeared from the local drugstore shelves. To overcome hurdles across patent-protected drug products and build local drug manufacturing capability to improve patient access, the EU Council adopted a regulation that provides an exception to an original molecule by providing a Supplementary Protection Certificate (SPC). The provision facilitates manufacturing of generics and biosimilars before patent expiration for stockpiling or export purposes. However, the exception will be applicable only under the following circumstances.
- The generics or biosimilars produced are exclusively for export to another country where the patent for the drug molecule doesn’t exist or has expired, or for stockpiling purposes within the outstanding patent term of six (06) months.
- Per the requirement, the manufacturer has conveyed the information to the local Health Authority and the holder of the SPC before three (03) months.
- The manufacturer has informed all the stakeholders concerned with the commercialization of the product.
- The manufacturer has ensured that the labeling requirements state that the drug product indicates that it is only for export purposes.
As a Matter of Fact
Since the grant of conditional marketing authorization for COVID-19 vaccine products in the EU, the European Medicines Agency’s Human Medicines Committee (CHMP) has authorized four (04) sites for vaccine production within a year; two (02) in Switzerland and two (02) in the USA. Such decisions in favour of expanding production sites will enhance vaccine manufacturing capabilities while making them more accessible to the European population.
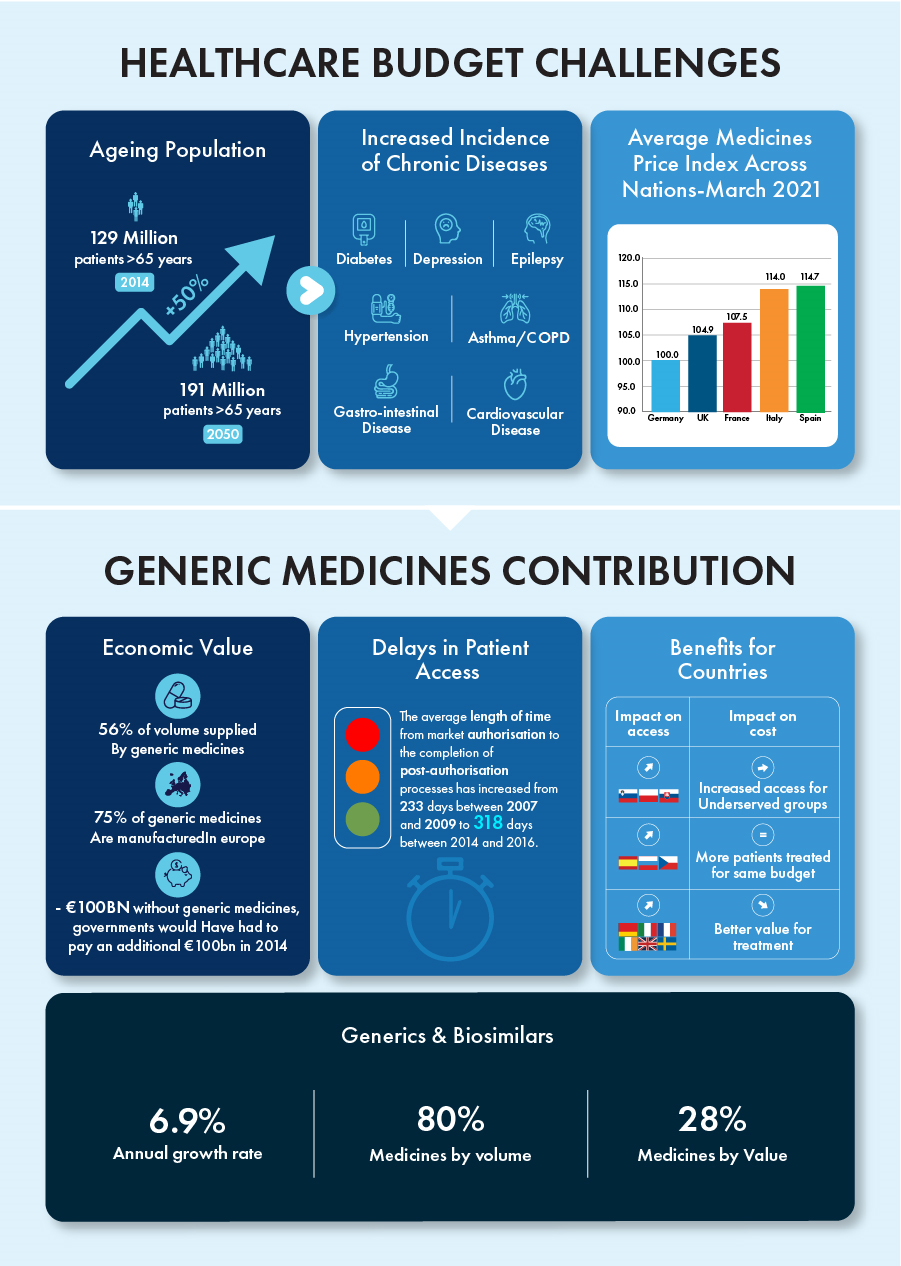
Generics and biosimilars have singlehandedly contributed to an annual growth rate of 6.9% while representing 80% of all medicines by volume and 28% by value in the past decade. The patent term for certain biologics valued at around €90 billion will end by 2023, creating a competitive environment for these products with tremendous growth potential.
Implementation of an SPC waiver would benefit the European Union by:
- Adding an additional €90.5 bn approximately per year.
- Generating twenty-five thousand (25,000) new job opportunities with two thousand (2,000) direct jobs for the EU API industry. The initiative will help EU member states save €3.1 bn on healthcare expenses.
- Stockpiling cheaper alternatives of innovator patented drugs to enable them to enter the market immediately after the Intellectual Property (IP) expiry, improving patient access.
For a drug manufacturer based out of the European Union, the SPC waiver implementation is one of the most rewarding initiatives in favor of generics. Since the EU Health Authority regulates medicines for human and veterinary use through a centralized or a decentralized procedure, navigating through specific local requirements can get overwhelming. An expert, aware of the local requirements regarding various submissions and license documents, can help you focus your efforts on essential production protocols rather than fixating on complex Regulatory roadblocks. Re-imagine your business potential by experiencing compliance and quality. Contact Freyr.









