
Competitive generics are known to be a boon as they pave the way to a lucrative market, provided drug manufacturers can decode the challenging and complicated drug development. Although drug development technologies are constantly evolving, we are yet to comprehend the outcomes of Competitive Generic Therapy (CGT). When we discuss the disease burden, the size and volume of the patient population demography have been critical elements for drug development and its approval. However, in therapy areas where generic competition is almost negligible, the cost of therapy invariably remains high due to the lack of competition in place. To facilitate competition in niche therapeutic areas post the United States Food and Drug Administration (US FDA) Reauthorization Act 2017, a drug can achieve an “inadequate generic competition” designation as a part of the CGT.
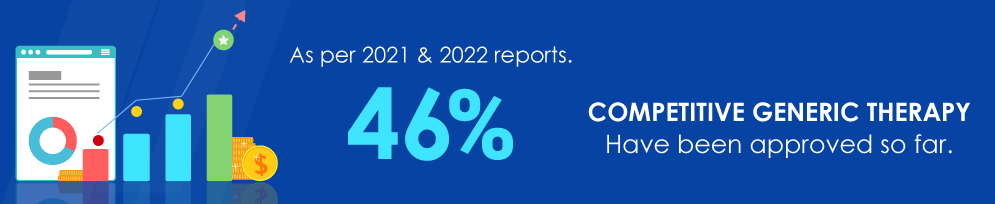
Data source: https://www.fda.gov/drugs/generic-drugs/competitive-generic-therapy-approvals
The US FDA is aware of the factors that may influence an applicant’s decision to develop a generic drug. For instance, drug "X" belonging to a niche therapeutic category may not attract a high level of interest from generic drug applicants when directly compared to drug "Y," which serves a larger patient population group. This may occur when there is a limited market for niche products and/or if the products are more difficult to develop. The difficulty of development can range from highly regulated markets to complex manufacturing procedures with a rise in demand for funds. If navigated correctly, niche therapy areas can play a significant role in diagnosing, treating, and preventing various types of diseases or conditions. The provisions in association with CGTs are intended to incentivize targeted development, efficient review, and timely market entry of drugs for which there is inadequate generic competition.
To facilitate increased competition for these products, the FDA may take certain actions to expedite the development and review of an Abbreviated New Drug Application (ANDA) for a drug designated as a CGT. These actions by the FDA may help to clarify the Regulatory expectations for a particular drug, assist applicants in developing a complete submission, and ultimately promote a more efficient and effective ANDA review process to reduce the number of review cycles necessary to obtain ANDA approval. An applicant can file a request for a drug to gain a CGT designation. The FDA describes the process by which a drug is granted the designation specific to a particular application and drug product, as interpreted in 506H. The CGT guidance provides a checklist to allow applicants to undertake a primitive level of self-evaluation.
Checklist to qualify for CGT exclusive designation:
- Presence of inadequate generics for a molecule by the Orange Book
- Request designation of a drug as a CGT
- Request for CGT designation to be stated before the ANDA submission
In a therapeutic segment where competition is inadequate, the unavailability of numerous brands to select from becomes a bottleneck. Time is of the essence to eradicate such bottlenecks in isolated therapy segments with critical patient treatment timelines. The FDA’s initiative to grant an innovator drug with a CGT ensures a continuous influx of essential or rare molecules in the market to bring down treatment costs. Experts at Freyr are familiar with the updated requirements stated as per the CGT guidance and would aid in identifying if the said pathway is the right fit for your drug. Experience perfection first-hand. Contact us today.









