
A warning letter is an official message/letter issued from a health authority to an organization that violates the set rules and regulations. When it relates to pharmaceutical companies, they need to follow several Good Manufacturing Practices (GMPs), GCPs, GDPs, etc., to avoid such warnings. There are several instances in which any Health Authority (HA), particularly FDA, issues these letters.
For example, recently, an Indian drug manufacturing company received a warning letter from the United States Food and Drug Administration (USFDA) for violations in its Active Pharmaceutical Ingredients (APIs) manufacturing facility. This action was based on the inspection of the facility carried out by the FDA a few months ago.
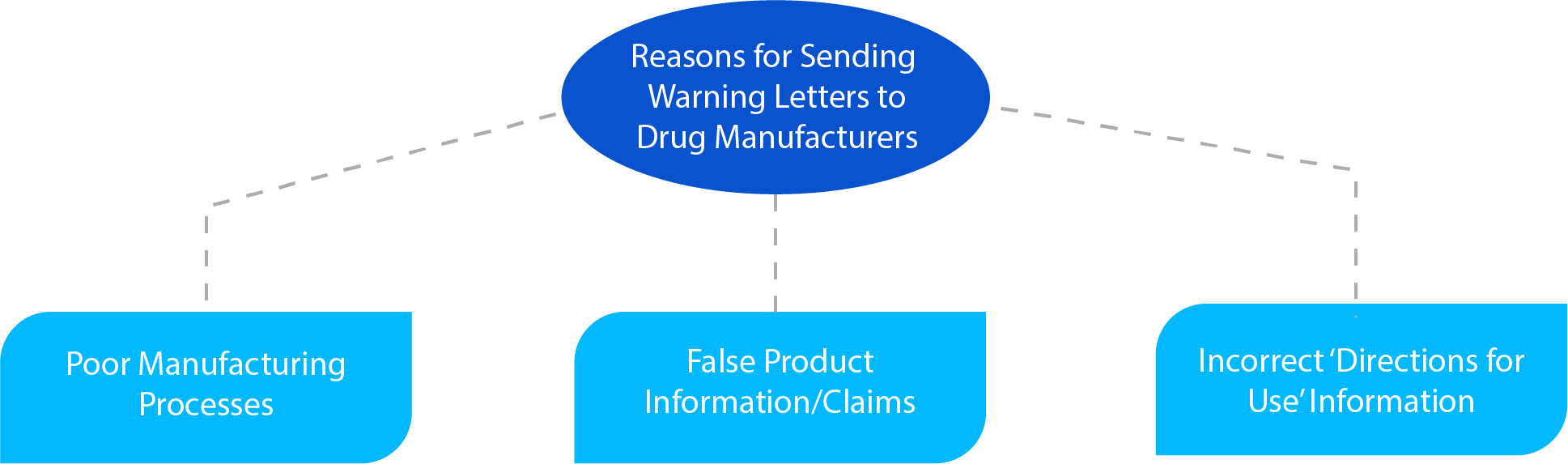
What a Warning Letter May Contain?
The warning letter is quite elaborative and may contain the following information depending on the case:
- The violation identified by the HA while conducting an inspection, essentially one of the three (03) reasons specified in the above diagram.
- List of corrective measures to be taken for the identified problem.
- Timeframe within which the manufacturer has to inform the FDA of the correction plan.
A warning letter, however, is a setback that can affect several factors in the business. Listed below are a few of them.
Repercussions of a Warning Letter
- Delay in time-to-market.
- Financial setback when taking corrective actions.
- Supply-chain liability owing to a halt in the manufacturing process.
Conclusion
Avoiding shortcuts and violations in the manufacturing lifecycle is the only way to avoid warning letters from the respective HAs. A few companies have had to shut down their operations owing to major breaches in the guidelines. The support from an end-to-end Regulatory expert like Freyr can help pharmaceutical manufacturers in following a compliant pathway. Reach out to Freyr now and avoid any risks.









