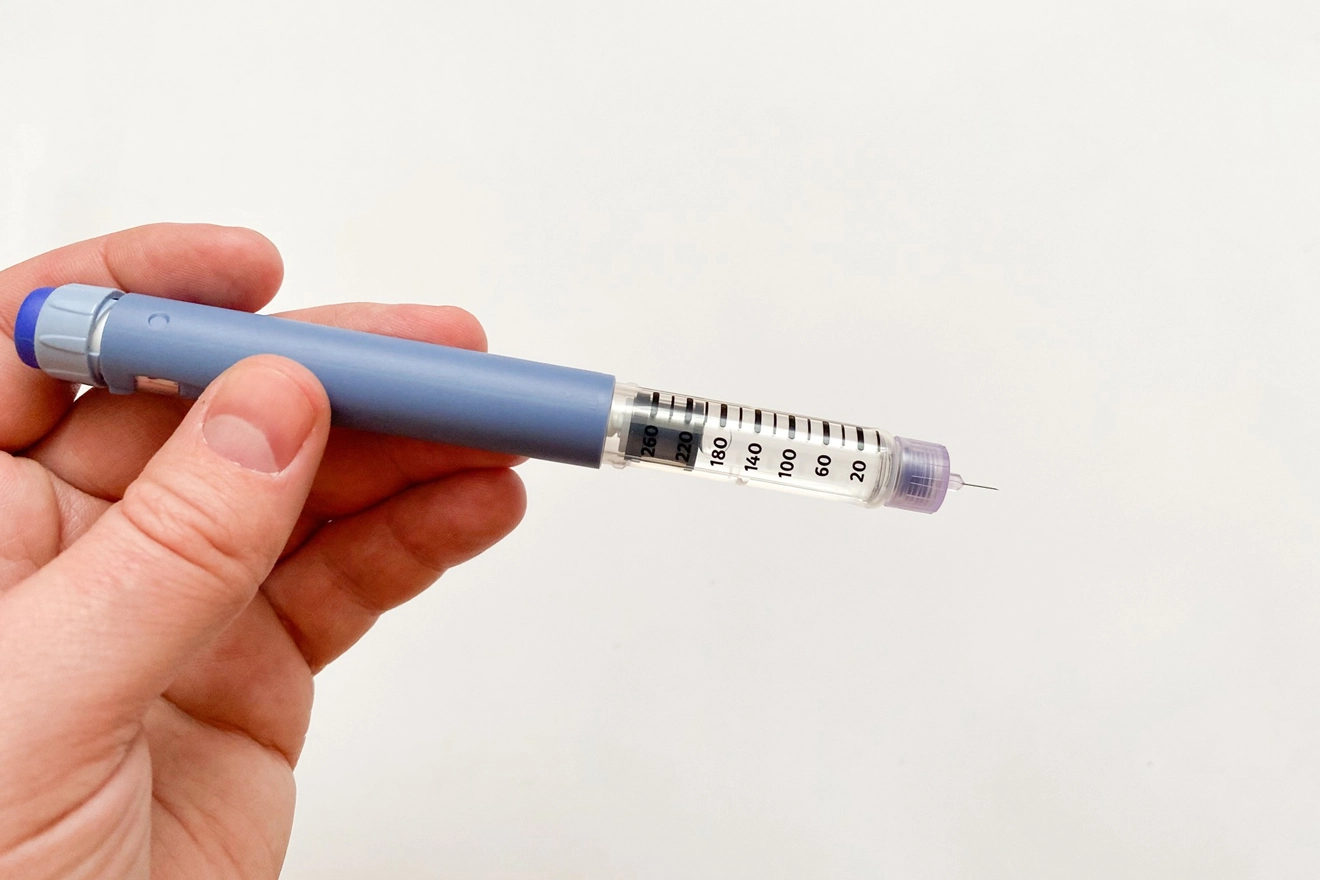
The customer is a US-based company specializing in reproductive health. The customer awaited to market their products (pre-filled pen injector for infertility treatment) in Japan and was seeking Regulatory support, including device component registration, approval, and gap assessment for PMDA compliance.
Download the proven case study to know how Freyr supported QMS implementation, provided reviews, and addressed all the setbacks in the process.
Fill the form below to download the Case Study



