Clinical Labeling Services - Overview
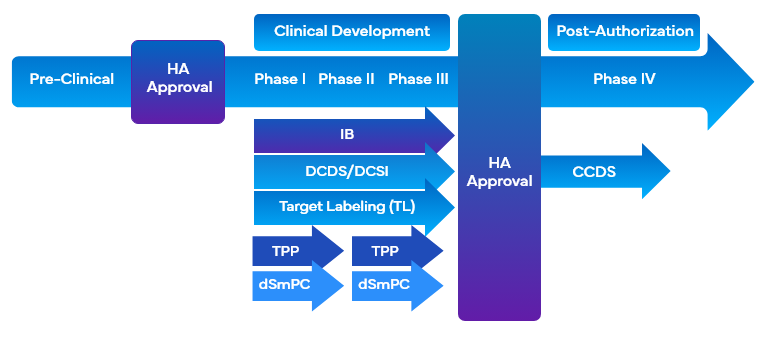
During drug development, companies utilize a variety of clinical labeling-related tools that employ target labeling to facilitate drug development. These tools simultaneously align the desired marketing and clinical trial labeling objectives with the development of program design or study design and facilitate the drafting of the Clinical Overview (CO). Some of the clinical labeling tools that utilize target labeling during drug development are:
- Investigator Brochure (IB)
- Target Labeling (TL)/Target Profile
- Development Core Data Sheet (DCDS)
- Development of Core Safety Information (DCSI)
- FDA Target Product Profile (TPP)
It is important for companies to employ a modular approach while collecting the safety and efficacy information across several types of clinical labels to complement the clinical labeling requirements. This would satisfy the need to help investigators and sponsors effectively by presenting and updating a focused and dedicated DCSI section that can conveniently be placed under different clinical categories.
Furthermore, DCSI is included in the Company Core Safety Information (CCSI), which is the basis for the first Company Core Data Sheet (CCDS) and is utilized for the product's entry into the market. Drafting a thoroughly versed CO comprising the scope and critical issues in the clinical developmental program about the drug is also required to support the documentation for the assessors.
Freyr’s Clinical Labeling Services
Clinical Labeling Services
Creation and Review of the Investigational Brochure (IB)
Freyr has considerable expertise in creating, authoring, and reviewing IBs for various clinical-stage programs for our clients. IBs are the most nascent forms of labels intended to provide the investigator or treating physician with relevant information regarding the drug/intervention. Their purpose is to give the investigators and others involved in the trial the necessary information to facilitate their understanding of the rationale for, and their compliance with, many key features of the protocol. These include dose, dose frequency/interval, methods of administration, and safety monitoring procedures. The IB contains pre-clinical and clinical information related to an investigational drug.

While working on IBs, Freyr’s clinical labeling experts present the information in a concise, simple, objective, and balanced form. The same qualities can be considered when translating documents as well. In addition to authoring IBs, Freyr also supports the annual review of IBs and works on revisions as necessary, in compliance with the standard procedures established by clients and sponsors.
Development of Target Labeling (TL)/Target Profile
Development and Review of DCDS & DCSI
Freyr has proven expertise in creating high-quality Developmental Core Data Sheets (DCDS). The DCDS is an intermediary core label vital in deriving content for first-to-file national labels (like USPI, SmPC, etc.). The DCDS/DCSI is prepared from the target label, and it provides integrated safety and efficacy to an intervention or drug. The DCDS/DCSI helps investigators and sponsors by presenting and updating focused and dedicated DCDS sections that can conveniently be placed within the IB. The DCSI facilitates the development of CCSI, which later forms an integral part of the Company's Core Data Sheet (CCDS).
Development and Review of FDA Target Product Profile (TPP) and EU-draft SmPC (dSmPC)
Target Product Profile (TPP)/draft Summary of Product Characteristics (dSmPC) is a form of clinical label that facilitates discussions between pharmaceutical companies and health authorities. The TPP/dSmPC is used throughout the drug development process, from pre-Investigational New Drug (IND) application and Investigational New Drug (IND) application phases of drug development through post-marketing programs, to pursue new indications or other substantial changes in clinical trial labeling. Pharmaceutical companies specify the labeling concepts that are the goals of the drug development program in the form of TPP/dSmPC. TPP/dSmPC provides a statement of the overall intent of the drug development program and gives information about the drug at a particular phase of development. As a strategic Regulatory partner, Freyr has expertise in preparing TPPs for the US and dSmPc for the EU.
- Resources with in-depth Regulatory knowledge in clinical packaging and labeling
- Expertise in successfully handling global and regional drug labeling for Fortune pharma clients in the USA, EUROPE, APAC, MENA, etc.
- Global Regulatory expertise in helping life sciences organizations, viz. pharma, biotech, and nutrition manufacturers
- Highly qualified medical writers possessing extensive Regulatory labeling experience
- In-depth and updated understanding of the global drug labeling changes from multiple health authorities, such as the US FDA, EMA, TGA, etc.
- Dedicated compliance team tracking the status of core and company core data sheet (CDS/CCDS) implementation in regional labels
- Expertise in clinical trial labeling and clinical labeling services
- Highly experienced pharmaceutical, biotech, and nutrition labeling professionals








