Cosmetic Regulatory Consulting
Freyr provides a range of cosmetic Regulatory services tailored to the needs of large, small, medium, and very small businesses. With our team of local experts, we offer comprehensive support for Regulatory Affairs in the cosmetics industry, ensuring smooth market entry across the globe. If you require assistance in cosmetic Regulatory compliance and quick turnaround services for global markets, we have the right expertise to meet your needs.
Industry News
Cosmetic Regulatory Expertise
Navigating Regulations, Ensuring Innovation: Your Trusted Cosmetic Regulatory Experts
Formulation & Ingredient Review
Evaluating and analyzing the composition and components of products to ensure safety, efficacy, and compliance with Regulatory standards.
Labeling Review
Assessing and verifying the accuracy, completeness, and compliance of product labels with Regulatory requirements and industry standards.
Claims Review
Evaluating and scrutinizing the accuracy, validity, and Regulatory compliance of product claims made in marketing materials or advertisements.
Safety Assessment & Toxicology
Evaluating the potential risks and hazards associated with products or substances to ensure their safe use and compliance with Regulatory standards.
Global Regulatory Compliance and Consulting (GRCC)
Freyr’s GRCC program provides scalable, end-to-end cosmetic regulatory support, acting as an extended regulatory team for global brands.
Cosmetic Product Information File
Comprehensive documentation ensuring Regulatory compliance and safety for cosmetics, containing formulation details and safety assessments.
Go-To-Market & Legal Representative Services
Overseeing product launches and serving as the legal liaison to ensure compliance with regulations and protect the company’s interests.
Cosmetic Testing
Conducting assessments to ensure the safety, efficacy, and quality of cosmetic products through various scientific methods, including in vitro and clinical testing.
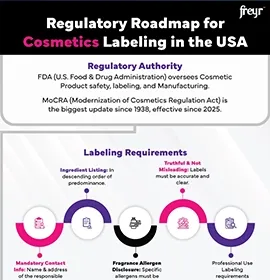
Modernization of the Cosmetics Regulation Act of 2022 (MoCRA)
Legislation enhancing regulations for the safety, manufacturing, and marketing of cosmetic products to meet the evolving industry standards and ensure consumer protection.
Cosmetic Regulatory Intelligence
Monitoring and analyzing Regulatory changes and trends in the cosmetics industry, which inform compliance strategies and decision-making processes.
EUDR Compliance Services for Cosmetics Industry | Freyr Solutions
Prepare for the EU Deforestation Regulation (EUDR) with Freyr's comprehensive regulatory services. Stay compliant and mitigate deforestation risks. Learn more now.
Global Cosmetovigilance Ensuring Regulatory Reporting & Post-Market Surveillance for Cosmetics
Comprehensive Sustainability Regulatory Services for Compliance, ESG Reporting, and Eco-Friendly Packaging Solutions
Global Regulatory Intelligence
Unveil global cosmetics compliance by blending strategic decision-making with comprehensive Regulatory Intelligence across ingredients, formulations, filings and beyond.
Country-specific Cosmetic Regulatory Consulting
Operating across continents, Freyr has established a strong global presence. With regional expertise and a deep understanding of local regulations, we deliver tailored solutions in key markets worldwide.
Africa & the Middle East
Contact Us for Cosmetic Regulatory Guidance and Support
Freyr's Approach to Cosmetics
At Freyr, we understand the intricacies of global cosmetic regulations, and we are here to guide you through the process of achieving Regulatory compliance for successful market entry. Addressing the product classification challenges, we navigate diverse jurisdictions with varying criteria, particularly in the complex realm of final product classification and ingredient assessment. Safety, a paramount concern, involves adhering to stringent standards and scientific principles. Furthermore, evolving regulations and consumer expectations towards green and sustainable products add an additional layer of complexity.
In regions with distinct Regulatory requirements for cosmetics, a robust market access strategy becomes essential to tackle procedural challenges in local regimes. Aggressive product claims are certainly one area that attracts frequent scrutiny. We have executed several compliance audits for large portfolios and support companies in mitigating gaps and risk.
Freyr excels in providing global cosmetic Regulatory Intelligence (RI) services, offering a Regulatory roadmap for compliance, and successfully guiding customers through expansion plans in burgeoning beauty markets. Our specialization encompasses:
- Product Registration
- Regulatory Compliance
- Safety Assessment
- Approvals and Notifications
- Legal Representation (LR) Services
- Labeling and Claims Assessment
- Regulatory Guidance
- Dossiers Preparation
- Regulatory Pathways
- Comprehensive Regulatory Strategies
In the cosmetic industry, ensuring Regulatory compliance for products is vital. A key step is meticulously compiling documentation required by Health Authorities (HAs), often in the form of a product dossier or a Product Information File (PIF). This comprehensive dossier and robust documentation requirements may cover product formulation, raw materials, ingredient data, labeling, testing results, and safety assessments.
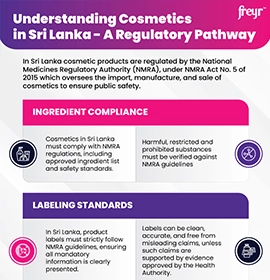
Cosmetic product classification, compliance, and registration rules vary in countries. Some mandate registration or notification, while others focus on compliance without formal registration. Terminology differs; some use simple notification or approval.
A thorough product dossier includes administrative, manufacturing, quality, control, and testing data. Freyr aids in understanding and compiling the technical documents needed for product registration in compliance with the HA regulations.
We streamline the process and offer assistance in gap analysis, document review, and compilation for successful Regulatory submissions.

For personal care products’ registration and notification, numerous countries require local support to facilitate effective communication regarding product status and safety for consumers. Addressing this need, companies seek a Legal Representative (LR) or Responsible Person (RP) to oversee continuous product compliance, handle queries, and manage post-market obligations. The representative acts as a vital intermediary between the manufacturer or brand owner and the Health Agencies (Has).
Freyr, with regional offices worldwide and an extensive partner network in 120+ countries, provides LR services for cosmetic products. Operating in Europe, North America, the Middle East, India, Asia Pacific (APAC), and beyond, we ensure seamless communication between customers and Has. Our services guarantee compliance with country-specific regulations, oversee product registration, and maintain product data validity throughout the specified period.

- Safety Assessment
Many countries require cosmetic products to undergo a rigorous safety assessment, including formula evaluation, exposure calculation, toxicological analysis of ingredients, and decision-making regarding safety compliance. Post-market monitoring ensures ongoing compliance and product safety. The process aims to safeguard public health by ensuring that personal care products and their ingredients meet safety standards before being introduced into the market and during the lifetime of the product.
Freyr supports the preparation of a Cosmetic Product Safety Report (CPSR) according to the guidelines of the respective HA, ensuring compliance. Upon completion, the reports are stored on behalf of the customer, with ongoing monitoring of legislative changes and, when required, Freyr follow-ups with the HA to address any inquiries or requests for additional information, facilitating a smooth market entry process.
- Product Testing
Testing represents an important part of a cosmetic product manufacturing process. Cosmetic products must undergo specific testing in order to be compliant, and more importantly, to prove they are safe and effective under reasonably foreseeable use conditions. A notable challenge is achieving consumer safety without animal testing, aligning with international Regulatory standards.
To uphold strict Regulatory standards and protect consumers, Freyr supports a range of cosmetic testing services, covering microbiological purity, challenge tests, stability, dermatological testing, efficacy, applicational testing, claims support, and sun protection testing. Our expert team assists manufacturers in navigating testing requirements and assisting with testing services with our partner laboratory network in Europe.

For a seamless entry into global markets, manufacturers need to comply with cosmetic label and claims requirements. Labels of personal care products are essential factors for the sales and marketing of cosmetics and should be carefully weighed to balance business objectives and Regulatory compliance.
With an expert Regulatory team, Freyr provides cosmetic label and claims review, label compliance, harmonization for multiple markets, translations, and substantiation support to achieve compliance and optimize market presence. Our comprehensive approach guarantees obtaining Regulatory excellence, distinguishing therapeutic claims from cosmetic claims, verifying claims support, ensuring global compliance, and boosting market success.