KGMP stands for Korea Good Manufacturing Practice which is based on ISO 13485:2016. It is the internationally accepted standard for medical device Quality Management System (QMS). All medical device manufacturers marketing their devices in South Korea shall comply with KGMP standards.
Is the KGMP Certificate Mandatory for all Devices?
The medical devices and In-Vitro Diagnostics (IVD) falling under Class II, III, and IV must comply with the KGMP regulations and shall obtain the KGMP certificate. The Class I devices are exempted from the KGMP certification but must adhere to the MFDS medical device GMP regulations. The Class I manufacturers may voluntarily opt for “GMP conformity recognition of Class I medical devices”.
What are the Statutory Bodies Involved in KGMP Inspection?
The class II devices are subjected to third-party review by MFDS accredited auditing bodies, whereas Class III and IV devices require combined inspection by the third-party reviewer and the MFDS.
Who Can Apply for the KGMP Certificate?
Domestic manufacturers can directly submit their applications to the third-party audit organization. For foreign manufacturers, the local authorized representative of the foreign manufacturer is required to obtain a license.
What is the Procedure for Obtaining a KGMP Certificate?
For the first time, the application (applicants- foreign manufacturer’s local AR and domestic manufacturers) must be submitted to MFDS accredited third-party reviewers. The process for obtaining a KGMP certificate is outlined below:
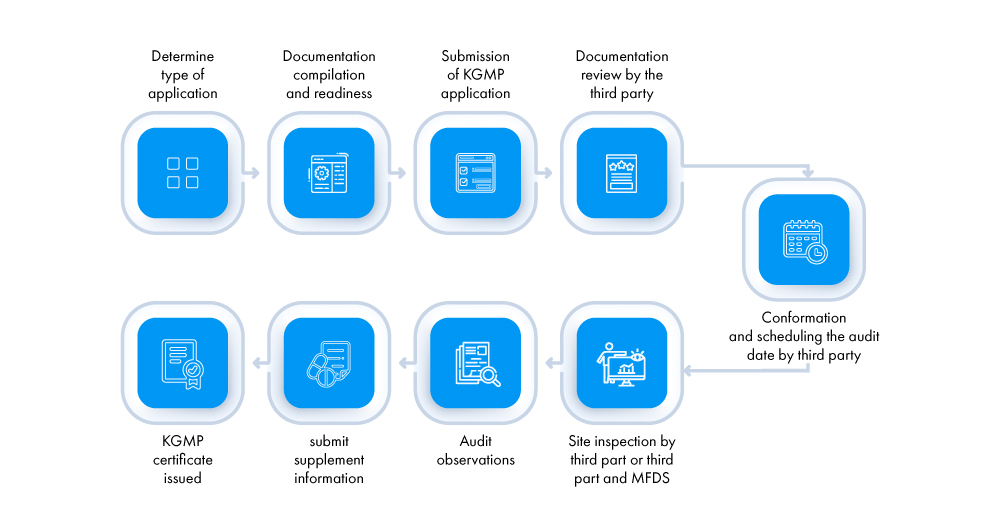
What are the Different Types of Inspections for Obtaining a KGMP Certificate?
Depending upon the device risk class and type of application (new, regular, or change), it can be either an on-site inspection or a documentation review. Before applying for KGMP certification, manufacturers must understand possible types of inspections and the applicable type for their scenario. The key factors to consider are:
- If any devices manufactured on the site are already registered and marketed in Korea or if the device in scope is the first to be launched in the Korean market
- If a new device (another category) is introduced in the already KGMP-certified manufacturing facility
- If the manufacturing site of the device is changed
Initial Inspection: Initial inspection is conducted when a device being launched is manufactured in a non-KGMP certified facility or if the existing KGMP certificate has expired before its renewal.
Regular Inspection: Regular inspection is performed every three (03) years for periodic assessment to ensure that manufacturers continue to comply with the GMP standards.
Additional Inspection: If a GMP-certified manufacturer adds and manufactures a medical device corresponding to a new category, it shall undergo additional inspection for the new product category. Based on the outcome of the regular inspection, an additional inspection can be either a partial or a full inspection. The validity of the certificate issued through additional inspection will be aligned with the initially issued KGMP certificate.
Change Assessment: Assessment is performed when the manufacturing site is changed. An exception is made for storage and laboratories, which have an insignificant impact on the product quality.
How is the KGMP Assessment Carried Out?
The manufacturing site will undergo either document review or on-site inspection by the third-party reviewers or MFDS.
Document review: Document review is performed when the manufacturer has a valid QMS certificate or when a valid site inspection report issued by certified agencies is available. When another importer wants to obtain a KGMP certificate for a manufacturer when it has a valid KGMP certificate from another import.
On-site inspection: On-site inspection is carried out for Class II, III, and IV medical devices. The site inspection can be carried out by third-party reviewers or can be jointly carried out by both the third-party reviewers and MFDS.
What are the Timelines for Obtaining a KGMP Certificate?
Depending on the correctness of the submitted document and the manufacturer’s preparedness for the on-site inspection, the MFDS issues the KGMP certificate within two-three (02-03) months of submission of the application. The timeline could extend further if the auditors raise any queries during the inspection, and manufacturers also might take a longer period to address the queries.
What is the Validity of the KGMP Certificate?
After a thorough inspection, MFDS issues a KGMP certificate which is valid for three (03) years and must be renewed after the period ends.
What is the Procedure for the Renewal of a KGMP Certificate?
If the KGMP certificate is due for expiry, the license holder can import the devices but should not distribute them until the KGMP certificate is renewed. The applicant shall submit the renewal application 90 days before the expiry of the KGMP certificate. The application will be taken up by MFDS accredited auditors, and the documentation presented during the three (03) years tenure of the original certificate will be reviewed. Once the inspection is completed, the KGMP certificate is renewed. The assessment for KGMP certificate renewal depends on the class of the devices.
Type of Inspection | Class II | Class III | Class IV |
Initial Inspection | On-site inspection by a third-party reviewer | Joint on-site inspection (third party and MFDS) | Joint on-site inspection (Third Party and MFDS) |
Regular Inspection | On-site inspection by a third-party reviewer* | On-site inspection by third-party reviewer* | Joint on-site inspection (third party and MFDS) |
Additional Inspection | Document review by the third party | Document review by the third party | Document review by the third party |
Change Assessment | Document review by the third party | Document review by the third party | Document review by the third party |
*Subjected to joint inspection in case of safety and efficacy concerns during the 3-year tenure
Manufacturers willing to enter South Korea must thoroughly understand the KGMP requirement put forth by the MFDS. Manufacturers are expected to always keep the detailed documents and the manufacturing site in compliance with the KGMP standards. Receiving the KGMP certification can be challenging for the manufacturers owing to the stringent Regulatory framework set up by the MFDS. The manufacturers can opt for an experienced Regulatory partner for appropriate strategy and obtain a KGMP certificate.
To know more about Korea's Good Manufacturing Practices, reach out to our Regulatory Expert.