About Us
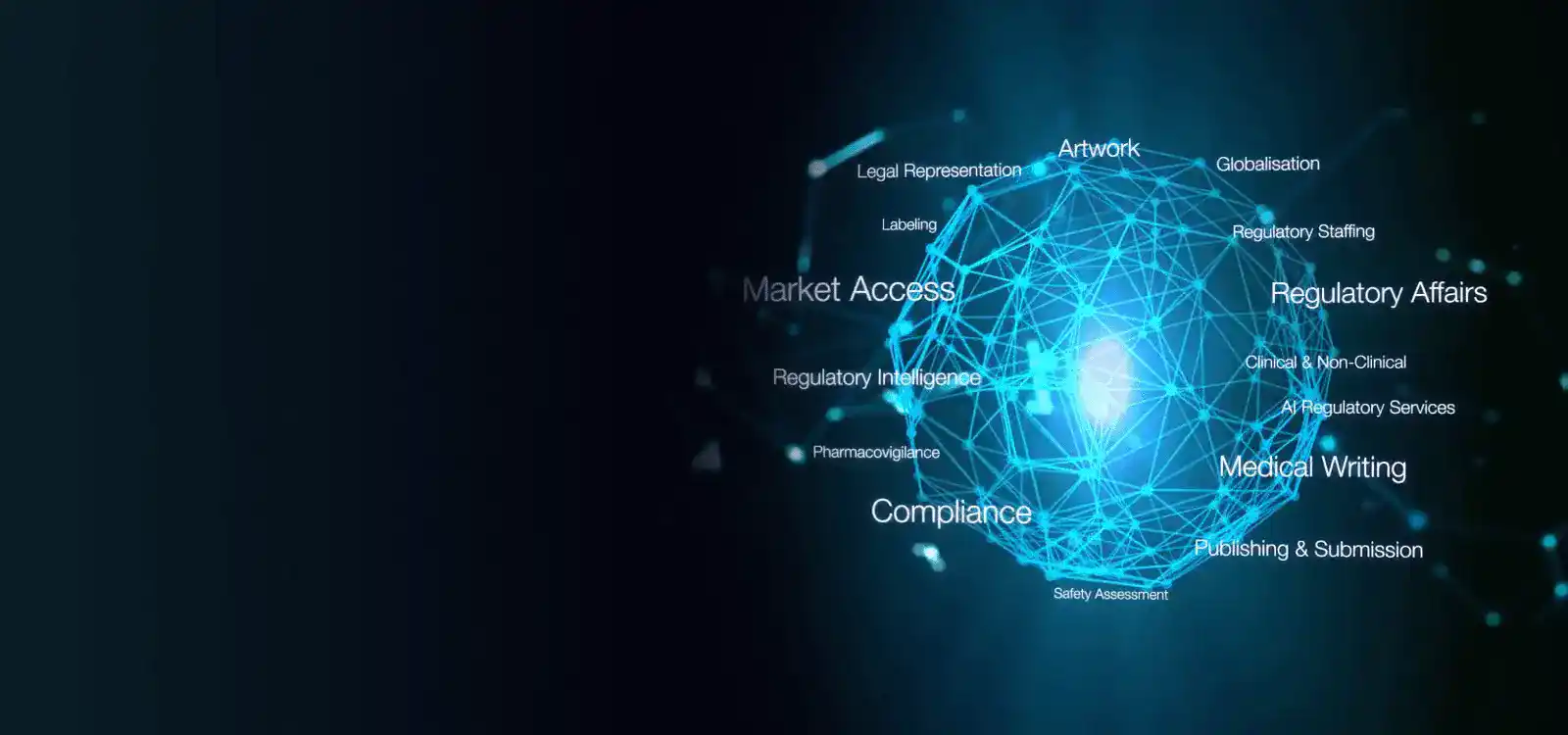
Freyr is the largest global Regulatory solutions and services provider, offering end-to-end solutions to the Pharmaceutical, Medical Devices, and Consumer sectors. At Freyr, we specialize in Regulatory Affairs, Pharmacovigilance, Quality Management, and Technology Solutions for Submissions Management, Labeling Management, Regulatory Information Management, and Regulatory Intelligence.
With our deep industry expertise, global presence, and commitment to innovation, we empower clients to navigate complex Regulatory landscapes efficiently and effectively. From Regulatory strategy to submissions and compliance, we are your trusted partner in ensuring product success and patient safety.
Success Stories
Powering Progress: Real Regulatory Wins, Transforming Compliance into Success!
Redefining Regulatory Excellence
Take advantage of our integrated suite of services, which range from end-to-end Regulatory consultation, process automation with robust Regulatory software, and harnessing the power of Artificial Intelligence (AI) in Regulatory intelligence, to transforming market entry challenges into growth opportunities.
Global Regulatory Services
Navigate Regulatory complexities effortlessly with our end-to-end global services. From strategy to compliance, we've got you covered globally. Ensure success in every market with Freyr Solutions.
Regulatory Software
Elevate your Regulatory efficiency with Freyr Regulatory Software. Streamline processes, ensure compliance, and conquer global markets seamlessly with our advanced Regulatory solutions.
Global Regulatory Intelligence
Stay ahead of the ever-evolving regulatory landscape with Freyr GRI. Powered by AI, NLP and ML technologies, our tailored and data-driven insights empower you to make informed strategic decisions with confidence and ease.
The First AI Regulatory Wiz
Celebrating Customers Success
We are proud partners to a diverse range of industry leaders. Our commitment to excellence in Regulatory services has earned us
the trust of esteemed organizations.
Functional Delivery Models
Shaping the future of Regulatory excellence!
Let Us Innovate and Succeed Together
Connect with Freyr, your Gateway to Regulatory Excellence!
Time is of the essence as we navigate the intricate world of regulations. Take control of your compliance journey today and allow our team of experts to guide you toward success. With our customized solutions, you would always be ahead in the constantly evolving Regulatory landscape, ensuring the growth and sustainability of your business. Let's collaborate and succeed together.