The application of software technologies in varied healthcare management, including diagnosis or treatment of a disease condition, is accelerating at an unprecedented rate. The global medical device Authorities are revamping their regulations and guidelines to address these booming device technologies. In Australia, Therapeutic Goods Administration (TGA) is the Regulatory Authority responsible for the oversight of medical devices, including software and mobile applications. In January 2021, the TGA has released the original draft on medical device software regulations, which was revised in the month of February 2021. On July 27, 2021, the TGA has released a detailed flow chart addressing the common ambiguities that device manufacturers and Regulatory professionals may have in relation to the Medical device software classification.
The TGA identifies the software used in the medical devices domain as Software as a Medical Device (SaMD), Software embedded within a medical device (SiMD), and Software that control medical devices. According to the TGA, the term Software as a Medical Device (or SaMD) is any software that can function on a laptop, smartphone, or tablet whose intended purpose falls under the norms of a medical device. In contrast, SiMD is a software that is an integral part of a device and is generally important for the accurate functioning of the device. Some software controls the medical devices either physically or via wireless connections like Bluetooth, Wi-Fi, etc. The TGA regulates all three (03) types of software.
Few medical device software are excluded or exempted from the TGA software as medical device regulation based on the principles of aligning the regulation with international regulation and reducing the burden through non-regulation of the products, where there is no major risk to safety, and suitable structures or framework for the product or system controls are already in place.
| Exempted Software Devices | Excluded Software Devices |
|---|---|
E.g., Few clinical decisions support systems that meet three main criteria: 1. If the software is not intended to analyze or process a medical image. 2. The software is intended to only provide support/recommendation to the healthcare professional. 3. The software is not intended to replace the clinical judgment of the professional. | The products that do not fall under the category of medical devices and are not subjected to any TGA Regulatory requirements are termed excluded software devices. E.g.,
|
The TGA has revised the regulation of medical devices comprising software that controls or interacts with other medical devices either externally or internally and the software which functions as a medical device (in its own right). The major changes include the introduction of new classification rules, illuminating the boundary of regulated software products, essential principles were updated to clarify the requirements for software-based medical devices.
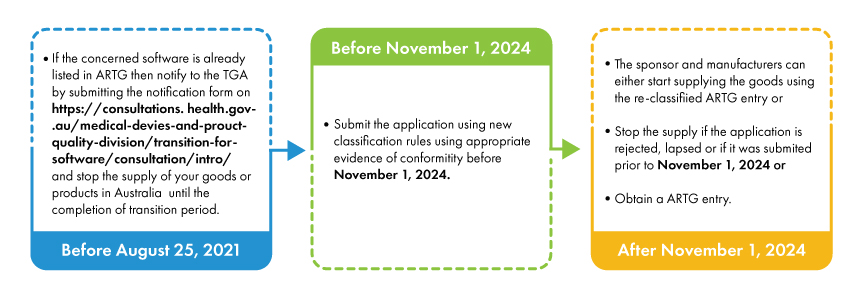
With the introduction of new classification rules, some software-based medical devices are being up classified into higher risk classes and must undergo relevant conformity assessment procedures. The sponsors and manufacturers of such up classified devices must be listed in ARTG (Australian Register of Therapeutic Goods) prior to February 25, 2021. The manufacturers and sponsors who have already applied to the TGA before February 25, 2021, must submit a notification form to the TGA.
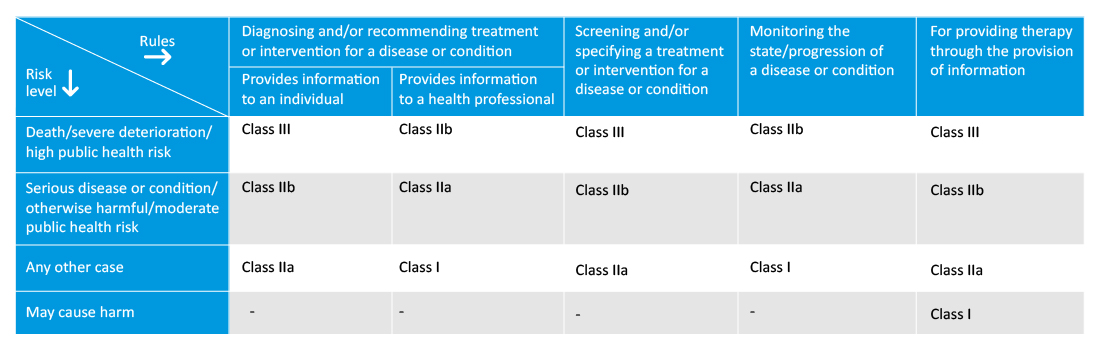
Medical device software classification in Australia:
Manufacturers must specify the intended purpose of the SaMD, which will help in further classification and conformity assessment procedures. The new regulation also introduces the new classification rules for programmed and programmable medical devices or software medical devices, and the class of the software will depend on the severity of the disease monitored/treated.
![]()
Amendments to essential principles:
Following are the changes made to the essential principles with effect from February 25, 2021, to the software-based medical device.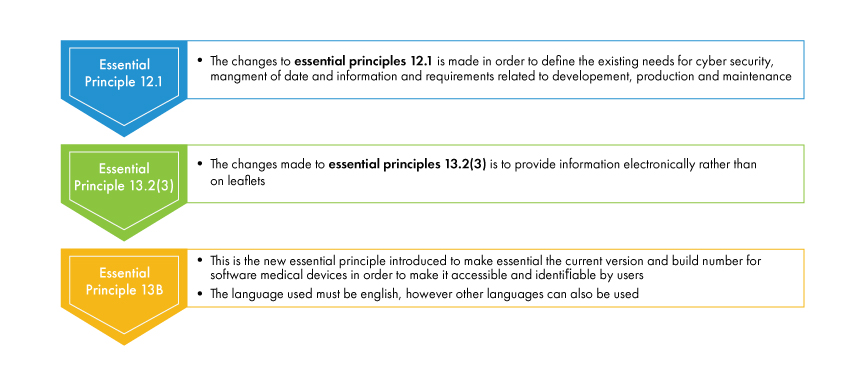
The Amendments done in the software regulations by the TGA can ease the burden of the manufacturer to categorize their software as regulated or non-regulated and can help in the preparation of documents required for the TGA conformity assessment on time.
With all the scenarios decoded, are you aiming to get your software listed in the Australian Register of Therapeutic Goods (ARTG) with less burden? For ARTG listing of medical devices, IVDs, or SaMDs reach out to an expert. Stay informed. Stay compliant.