
Innovations in drug-device combinations have led to significant advancements in patient care. Drug product(s) coupled with medical devices offer targeted drug delivery, improved therapeutic outcomes, and enhanced patient adherence. However, navigating the Regulatory landscape for drug-device combinations can be complex.
This blog will explore the considerations and challenges associated with the Regulatory operations for drug-device combination products, which offer insights into streamlining the processes.
Overview and Regulatory Framework for Drug-Device Combinations
Drug-device combination products synergize the therapeutic effects of pharmaceutical drugs with the delivery capabilities of medical devices. Drug-device combinations involve drug-eluting stents, inhalers and respiratory devices, transdermal patches, insulin delivery systems, drug-infusion systems, and drug-coated balloons. Targeted delivery, enhanced efficacy, improved patient adherence, and precise control over treatment are some of the notable attributes of these products.
Regulatory authorities like the United States Food and Drug Administration (US FDA), European Medicines Agency (EMA), and others have established drug-device combinations guidelines. Recent years have seen notable developments in the Regulatory landscape, with an increased focus on harmonization, risk-based assessments, and post-market surveillance.
Key Considerations for Regulatory Operations
When navigating the Regulatory operations for drug-device combinations, some of the considerations that come into play are detailed below:
Understanding product classification and the Regulatory pathways
Understanding the classification of drug-device combination products and selecting the appropriate Regulatory pathway is crucial. Manufacturers must determine whether it is a drug-device combination, drug-coated device category, or co-packaged product category. Selection of the correct pathway for the specific product type is vital for successful market access.
Addressing the scientific and technical requirements
It includes ensuring compatibility between the drug and device components, conducting stability studies to assess the product shelf life, validating manufacturing processes, and implementing risk assessments to identify and mitigate potential hazards.
Patient care and usability testing
Patient care and usability engineering play a significant role in such combination products’ development. Considering factors such as user interface, ergonomics, device handling, and patient adherence during product development helps ensure their safe and effective use by patients and healthcare professionals with minimal errors.
Post-market considerations
Post-market obligations are a critical aspect of Regulatory compliance for drug-device combinations. Manufacturers must establish processes for adverse event reporting, post-market surveillance, and vigilance reporting to monitor the safety and performance of their products.
Staying updated with recent Regulatory guidelines and updates
Keeping track of the recent guidelines and updates is crucial for ensuring compliance and aligning Regulatory strategies with the current expectations. The Regulatory landscape for drug-device combinations is constantly evolving. Aligning with the FDA, EU Medical Device Regulation (MDR), and the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines can help manufacturers stay compliant.
Best Practices for Streamlining Regulatory Operations
To optimize the Regulatory operations for drug-device combinations, pharmaceutical companies can follow the best practices illustrated in the pie chart below:
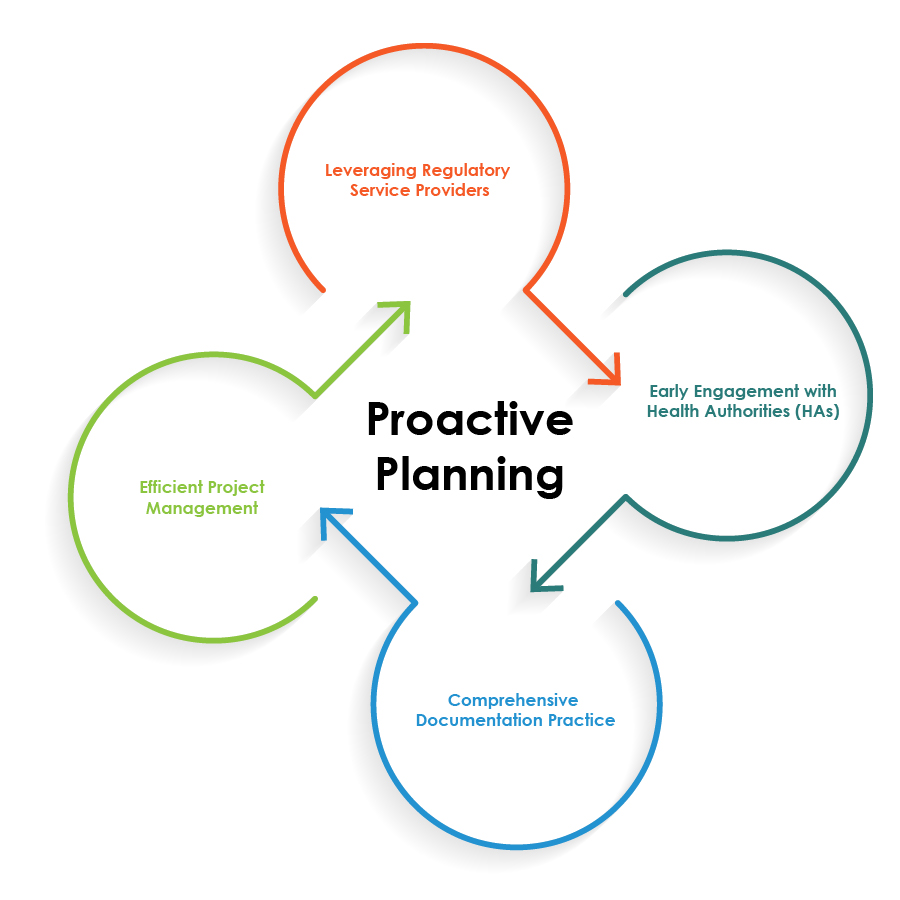
Proactive planning is the cornerstone for a seamless drug-device combination Regulatory operations
Future Trends and Challenges
Keeping an eye on future trends and shortcomings is crucial for pharmaceutical companies and Regulatory professionals involved in these products’ Regulatory operations. Listed below are a few trends and challenges to look out for:
- Emerging technologies: Nanotechnology, 3D printing, and allied applications influence the development of innovative drug-device combinations. These technological trends offer targeted drug delivery, personalized medicine, and enhanced patient monitoring. However, they also present unique Regulatory challenges related to safety, efficacy, and quality control.
- Cybersecurity: With the increasing connectivity of medical devices, ensuring data security and protecting against cyber threats becomes paramount. Integrating robust cybersecurity measures and staying updated on best practices for device security are critical considerations for Regulatory operations.
- Miscellaneous challenges: These include global supply chain complexities such as sourcing raw materials, component availability, and manufacturing processes, which pose challenges in maintaining Regulatory compliance and ensuring product quality. Robust supply chain management practices, risk assessments, and supplier oversight are essential to address these challenges and mitigate the associated risks.
Navigating the Regulatory landscape for drug-device combination products requires a comprehensive understanding of the Regulatory framework, recent guidelines, and best practices. Addressing key considerations, staying up-to-date with changes, and implementing best practices will aid pharmaceutical companies in streamlining their Regulatory operations. Collaborating with Regulatory vendors allows companies to leverage specialized knowledge and experiences and optimize their chances of Regulatory success in this evolving and demanding field. With our proven expertise in adhering to global Regulatory norms, Freyr can help achieve a compliant product journey. To know more, contact us.









