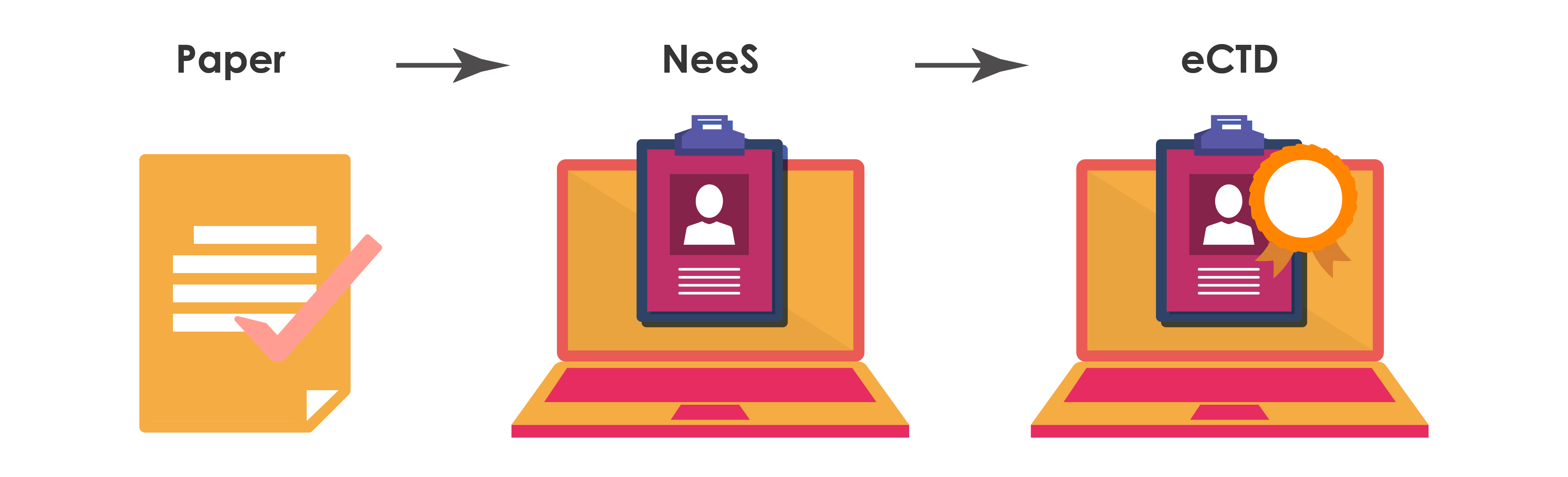
Electronic submissions have taken the life science industry's Regulatory space by storm. Majority of the Regulatory submissions are now made using the eCTD format globally. The benefits of eCTD are undeniable - faster, more efficient, and standardized. But transitioning from non-eCTD electronic Submission (NeeS) to eCTD isn't always a cakewalk. In this blog, we'll dive into the benefits of eCTD, the challenges of transitioning, and some strategies to successfully transition to the electronic world of submissions.
Benefits of eCTD:
eCTD provides several benefits over NeeS. One of its primary advantages is that the use of eCTD format reduces the time and resources required for Regulatory submissions. It also provides better tracking and management of Regulatory information, allowing companies to easily track the status of their submissions and ensure compliance with Regulatory requirements.
eCTD Transitioning and Challenges
Transitioning from NeeS to eCTD can be challenging. One of the primary challenges is the potential time and resource costs for the transition. Companies may need to invest in new software, hire additional staff, and allocate additional resources to the submission process. Additionally, employees may need training and education to ensure they are familiar with the new format and submission requirements.
Strategies for a Successful eCTD Transition:
To ensure a successful transition from NeeS to eCTD, companies should consider 3 key strategies.
First, understanding and involving all important stakeholders is necessary for a smooth transition. This may include representatives from Regulatory affairs, IT, and other relevant departments.
Second, is to invest in training and educating employees. This can include in-house training sessions or online training courses. By providing employees with the knowledge and skills they need to use the new format, companies can minimize disruptions to the submission process.
Lastly, companies should consider utilizing a software. There are several software solutions available that can help companies manage their eCTD submissions more efficiently. These solutions can automate many submission tasks, reducing the risk of errors and inconsistencies.

Transitioning from NeeS to eCTD can be challenging, but the benefits of the switch are significant.
In a nutshell, the primary goals of eCTD are to execute changes that fasten the Regulatory submission process, enhance the communication between Agencies and sponsors, and improve global synchronization of the format. An appropriate eCTD vendor can be the right fit for an organization to bring these changes. #findyourectdpartner by investing in the right strategic partner to accelerate your new product launches.
With this thought, we at Freyr, being at the forefront of driving innovation through advanced tech-enabled products, will be geared towards supporting our customers by adopting eCTD, in our Regulatory submission and publishing software - Freyr SUBMIT PRO.