
To enhance the accessibility of innovative medicines for patients in the UK post-Brexit, a new and accelerated approval pathway is known as the Innovative Licensing and Access Pathway (ILAP) was launched in January 2021. Its primary objective was to provide an integrated roadmap for all the stakeholders that enhances the process of product development. ILAP further supports commercial and non-commercial developers by facilitating the access of innovative medicines to the patient population by reducing the time-to-market. Pharmaceutical companies can explore ILAP as a route once the non-clinical data is consolidated. It is applicable for New Chemical Entities (NCE), biological medicines, new indications, and repurposed medicines.
The ILAP is an initiative developed jointly by the Medicines and Healthcare products Regulatory Agency (MHRA) in association with the All-Wales Therapeutics and Toxicology Centre (AWTTC), the National Institute of Health and Care Excellence (NICE), and the Scottish Medicines Consortium (SMC) as permanent partners. Supporting partners in the ILAP include the National Health Service England (NHS), the Health Research Authority (HRA), and the National Institute for Health Research (NIHR). Together, the stakeholders impart enhanced Regulatory, scientific, ethical, and commercial inputs in the early stage of medicines’ development during clinical trials.
In Freyr’s opinion, the ILAP offers great opportunities for developers of innovative treatments, especially to those who address unmet patient needs. For a relatively modest fee (approximately £8000), a company could secure early-stage advice, gain a designation for their product, and agree on a target development profile. This has the potential to save a significant amount of time from the overall product development lifecycle. There are also benefits in looking beyond marketing authorization and making early preparations for negotiations on reimbursement.
The ILAP compares favorably with similar schemes offered within the European medicines network (such as the PRIME). Whilst different in concept and delivery, the ILAP has broader acceptance criteria and a higher rate of acceptance. The ILAP also provides a path for integration with the United States Food and Drug Administration's (USFDA’s) Project Orbis for emerging oncology products.
How to Apply for the ILAP?
To access the ILAP, applicants must apply for an Innovation Passport (IP). Securing an IP designation enables drug developers to access the pathway based on the evidence required for a product to comply with the eligibility criteria defined by the NICE, SMC, AWTTC, and the MHRA.
This designation acts as a catalyst for innovative products from the pre-clinical to the mid-development stage. The IP can lead to the definition of a Target Development Profile (TDP) document. The TDP is a roadmap that facilitates access to the ILAP partners at early stages during the drug development lifecycle to accelerate and expedite access for patients to new and innovative treatments. Patients are a part of this entire process. Several tools provided by the ILAP support applicants at all stages of the design, development, and approvals process.
Innovative Passport Designation Criteria
The MHRA guidance clears the criteria for an IP designation. This is particularly relevant to innovative products, and it also compares favorably with equivalent schemes offered in Europe.
In the future, specific applicability of the IP for medical devices and combination products, areas in which there is always significant innovation, can prove to be beneficial.
The IP designation is evaluated by the permanent and supporting partners based on the following criteria:
- Criteria 1: Details of the condition, patient, or public health area
- For life-threatening and serious conditions
- In an event where the need is crucial to patients
- Criteria 2: The medicinal product fulfils one or more specific areas that include
- Innovative medicine
- Medicine associated with a new indication
- Medicine for rare disease
- Medicine under development for the objective of UK public health
- Criteria 3: The medicine has the potential to offer
- A brief on how the proposed medicine or indication will benefit the patients.
- Views from patients or patient organizations are encouraged.
On submitting the IP Application, a meeting between the applicants and the MHRA takes place to understand if the product fulfils the three (03) criteria.
As per the latest information in January 2022, the MHRA has received seventy-one (71) applications of which forty-one (41) resulted in the IP designation, twenty-two (22) are being processed, and seven (07) applications have been declined.
The Right Time to Enter the ILAP is mentioned below.
- At the early to mid-development stage of the product
- When relevant data is available
- When the applicants feel the need to get inputs from the stakeholders
- The applicants aspire to adopt new innovative approaches
- Not when the product is towards the end of the development stage
Overview of Medicine Development
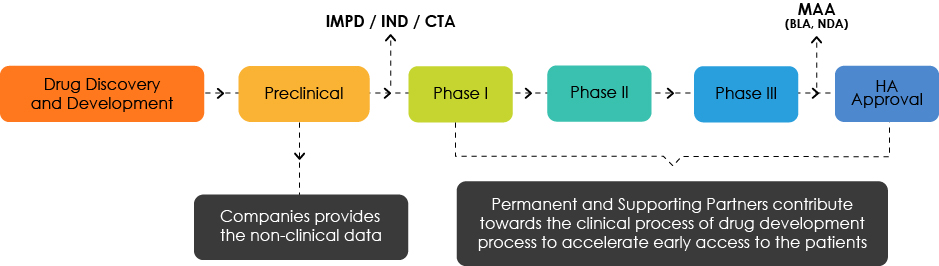
Understanding the Target Development Profile (TDP)
The TDP experts analyze the product characteristics and highlight shortcomings from a Regulatory standpoint. They are responsible for hinting the drug developers towards a roadmap that delivers early patient access to their products.
TDP is a document that is updated on a real-time basis per the requirements aligned with product development. Hence, a product undergoes multiple TDP amendments as new data gets generated.
Patient accessibility to essential medicines can be compromised by market delays. To avoid such scenarios, accelerated pathways function as a catalyst. The ILAP is an example of a flexible authorization pathway that is well-accepted across the industry and can hasten the development timeline of innovative medicines. The detailed criteria defined by the UK’s Healthcare System allow applicants to explore their eligibility to apply for IP designation. Incentivizing unmet clinical and patient needs with accelerated pathways like the ILAP facilitates market entry and can provide relief to patient populations at risk. To safeguard the interest of patients, Health Authorities do not compromise on the safety, efficacy, or quality of the product while ensuring an expedited approval process. Freyr’s end-to-end Regulatory services enable ease of documentation process to support the Innovative Passport designation in an expedited time, helping your products to reach patients earlier. Contact Freyr.









