In the dynamic realm where medical breakthroughs intersect with stringent Regulatory frameworks, the journey of clinical trials unfolds. At a critical juncture where innovation converges with compliance, clinical trials are the conduits through which transformative therapies reach the hands of those who need them. At the epicenter of this intricate dance is the evolving role of Regulatory vendors. Once considered mere service providers, they have now metamorphosed into strategic partners, reshaping the contours of clinical trial management. This expansive exploration delves into the transformative prowess of Regulatory vendor partnerships, dissecting how these collaborations not only ensure compliance but also elevate the efficiency, resilience, and success of clinical trials.
Navigating the Complexities of Clinical Trial Compliance
Clinical trials are a linchpin of progress in the pharmaceutical landscape. Yet, their management is fraught with complexities stemming from rigorous regulations and ever-evolving standards. Ensuring adherence to these standards is not just a Regulatory checkbox; it's an indispensable factor that dictates the trajectory of a trial. As complexities multiply, so does the necessity for a more strategic and proactive approach to compliance.
Redefining Vendor Relationships: Beyond Service Providers
The paradigm shift in the relationship with Regulatory vendors is not merely semantic but foundational. They have transcended their roles as transactional service providers, evolving into strategic collaborators actively shaping trial design, execution, and success. This shift signifies a departure from transactional engagements toward partnerships characterized by shared goals and a mutual stake in achieving compliance excellence.
Crucial Components of Regulatory Vendor Partnerships:
- Early Engagement for Holistic Planning
The journey of a Regulatory vendor partnership begins at the planning stage. Regulatory vendors engage in strategic discussions alongside pharmaceutical companies, weaving compliance considerations into the very fabric of trial planning. This early involvement lays the groundwork for aligning trial strategies seamlessly with Regulatory requirements.
- Risk-Based Approaches
Central to the partnership is a holistic, risk-based mindset. Regulatory vendors, through meticulous risk assessments, identify potential stumbling blocks in the Regulatory journey. This allows for preemptive actions to mitigate risks and prevent deviations from compliance standards, fostering a proactive rather than reactive compliance strategy.
- Real-Time Compliance Monitoring
Compliance is not a static state but a dynamic process that requires continuous vigilance. Regulatory vendors offer real-time insights into the compliance status of the trial. This real-time monitoring allows for prompt adjustments, ensuring that any deviations are addressed promptly, minimizing the risk of non-compliance.
- Tailored Regulatory Strategies
Recognizing the uniqueness of each trial, Regulatory vendors craft bespoke Regulatory strategies. This customized approach ensures that the strategies in place are not only compliant but also optimized to the specific context of the trial. It's a departure from one-size-fits-all solutions to tailored, precision strategies.
Impact on Clinical Trial Efficiency and Success:
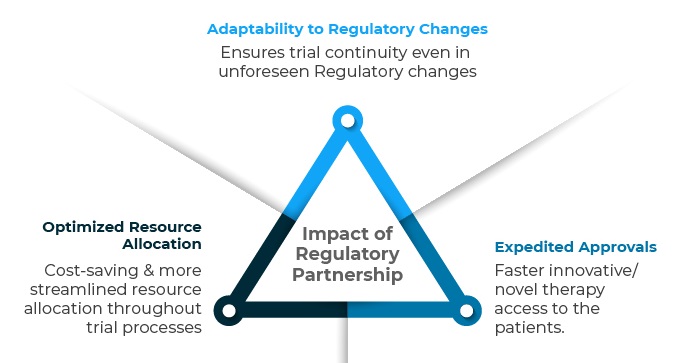
Impact of Regulatory Partnership goes beyond imagination amidst dynamic Regulatory norms.
A Synergetic Approach to Clinical Trial Management:
In conclusion, Regulatory vendor partnerships herald a new era in clinical trial management. It's a departure from the reactive stance of merely meeting compliance standards to a proactive, synergistic collaboration that enhances overall trial efficiency. As pharmaceutical companies and Regulatory vendors join forces, clinical trials become more than just a Regulatory obligation; they transform into strategic initiatives that drive innovation and redefine the future of healthcare.
Regulatory vendors emerge as the architects of a future where Regulatory complexities are not barriers but gateways to unparalleled advancements in healthcare. It's not just about compliance; it's about navigating the future with a collaborative spirit.