
The introduction of high-quality and reasonably-priced generic drugs in the market is a prevalent challenge for multiple Health Authorities. Several countries are taking steps to address this issue and make more generic drugs available in the market, consequently increasing the competition at a global level.
Let us talk about the United States pharma market. Developing generic drugs is a hard enough process. Additionally, manufacturers have to ensure the Abbreviated New Drug Application (ANDA) submissions meet all the prescribed requirements for faster approvals. To make it easier, the Food and Drug Administration (FDA) announced the Drug Competition Action Plan (DCAP) in 2017 to enhance the competition in generic drug development.
With the DCAP, the FDA aims to remove the barriers to generic drug development by enabling faster market entry so that they are available to needy patients at affordable prices. While the time-to-market is reduced with this Plan, the FDA also ensures the safety and efficacy of the generic drugs with the help of a transparent and efficient drug review process.
Significant Features of the DCAP
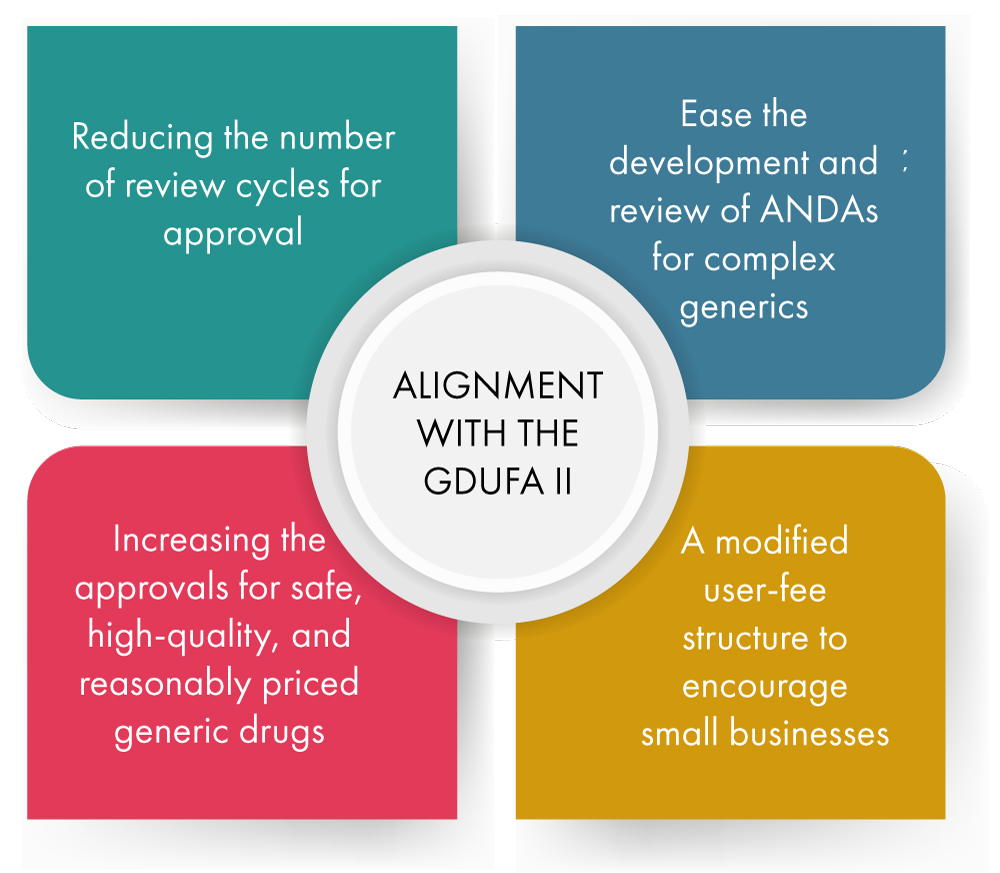
Alignment with the Generic Drug User Fee Program (GDUFA) II - Below is a figurative explanation of how this is being accomplished.
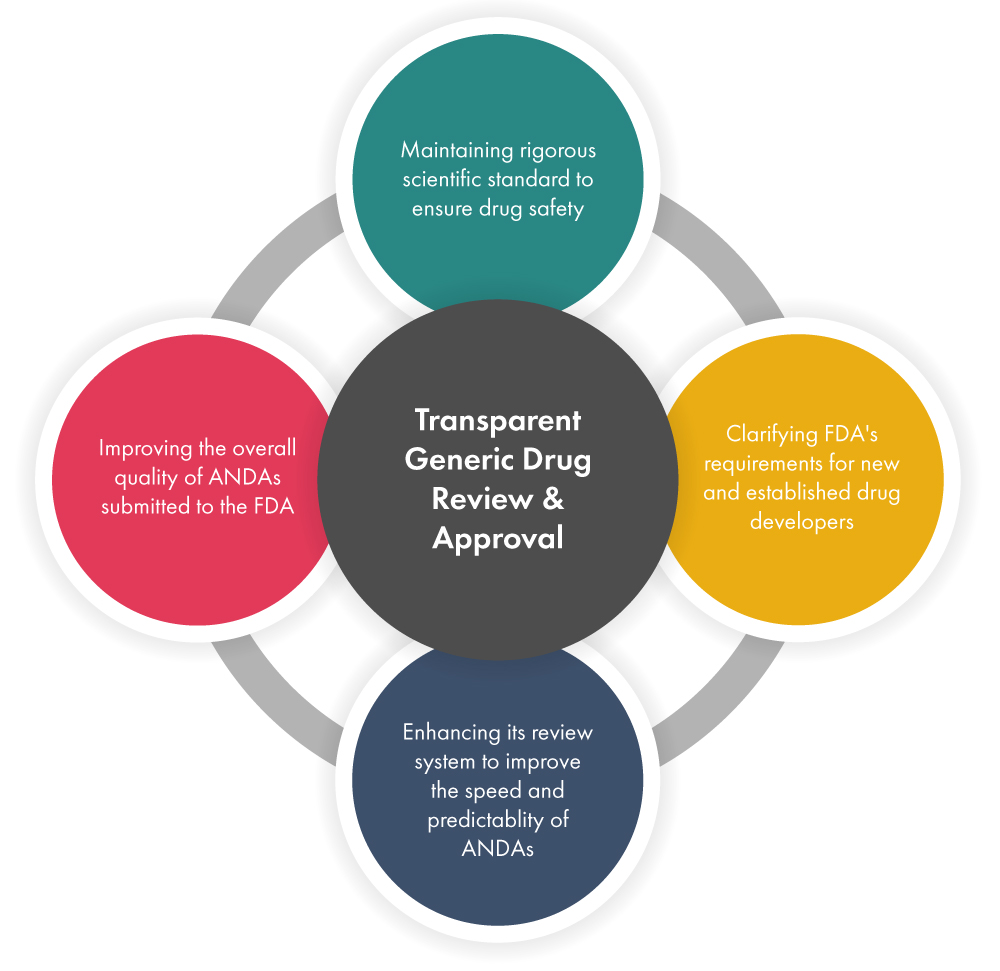
Enhance the generic drug development efficiency while hastening the review and approval process – The FDA is taking several initiatives to ensure that the generic drug’s review and approval process meets all the safety standards before bringing them to the market faster. Below is a representation of how this is done.
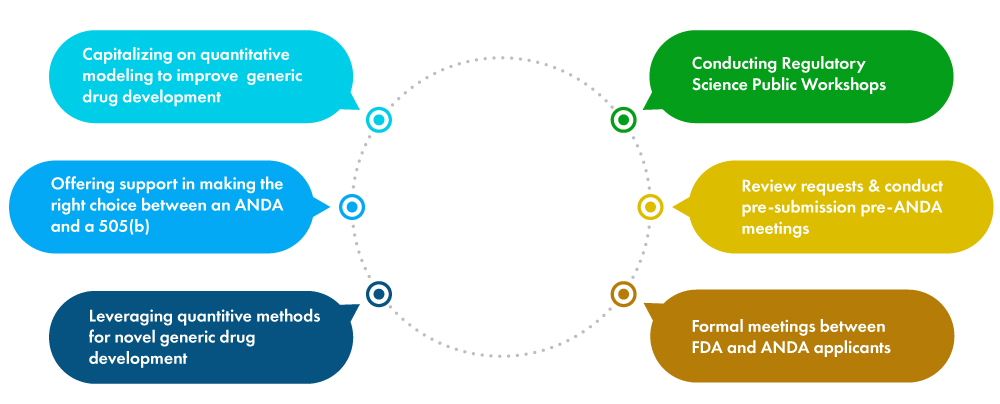
Increase the scientific and Regulatory clarity on complex generic drugs – It is a fact that complex generic drugs are harder to develop when compared to generic drugs. Consequently, the competition for such drugs is much lesser. Since the DCAP proposes to increase competition in the generic drugs market, the FDA has issued several guidance documents to generic drug manufacturers. A few of them are listed below.
Address the loopholes that allow innovator drug manufacturers to delay the generic competition that Congress aims for with the DCAP - This method used by brand-name drugs is also called ‘gaming’ by the FDA. It delays the generic drug approval process, and subsequently, reduces the competition. The Agency aims to streamline the development and approval process by making it predictable and transparent.
Conclusion
The main aim of the DCAP is a reduction in the monopolization of brand names when it comes to generic drug development and approval by creating an efficient process while ensuring their quality. Patients do not have to spend for costly brands as they have access to lesser-priced and effective generic drugs readily available in the market.
The FDA is releasing new guidance documents for the industry to this effect, and generic drug manufacturers must be aware of them and follow them to guarantee faster approvals. A proven Regulatory partner like Freyr can help you stay up to date with the latest regulations. Consult Freyr for compliance best practices.









