In the pharmaceutical industry, Regulatory compliance is of utmost importance. You must ensure that product labels, packaging, and the accompanying artwork adhere to stringent Regulatory standards, which is essential for meeting legal requirements and maintaining patient safety.
To navigate this complex landscape, companies often seek the assistance of Regulatory artwork service providers. These specialized vendors offer services in designing, reviewing, and managing artwork assets to help ensure compliance.
In this blog, we shall explore the key factors you need to consider when choosing a Regulatory artwork service provider in the pharmaceutical industry. Figure 1 below provides an overview of the factors.
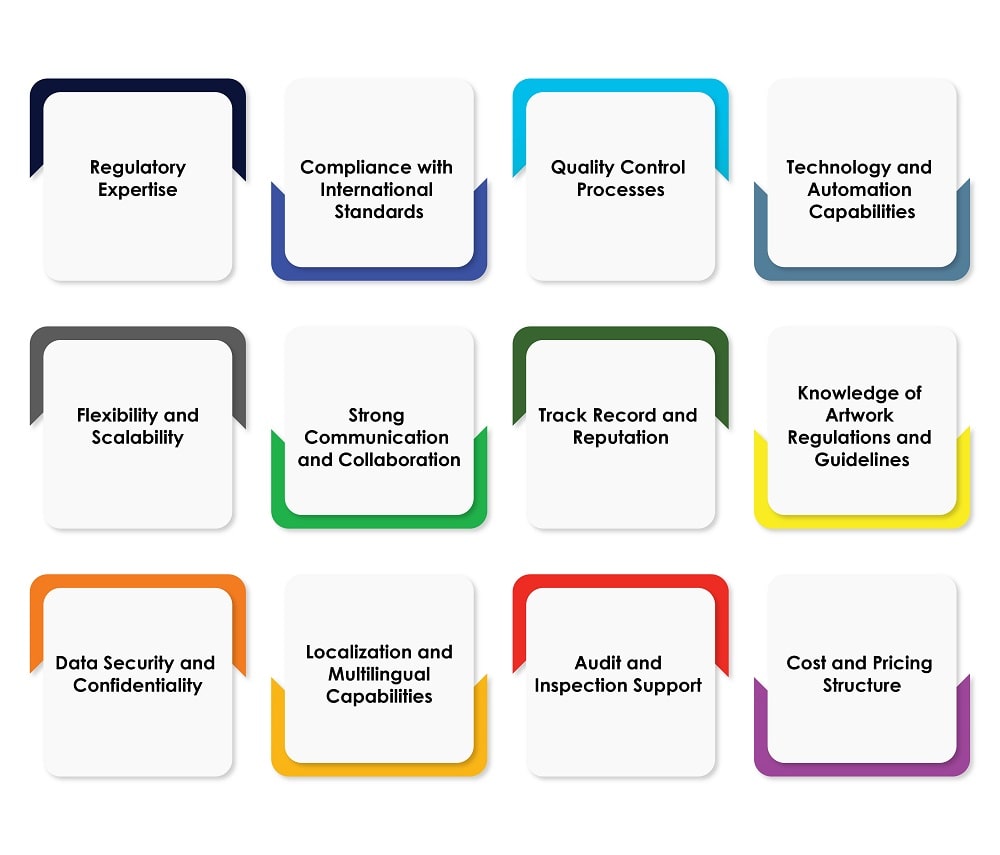
Figure 1: Considerations When Choosing a Regulatory Artwork Service Provider
Let us now delve into the factors outlined above:
- Regulatory Expertise: The pharmaceutical industry is heavily regulated, with numerous guidelines and requirements in place. In selecting a Regulatory pharma artwork service provider, prioritize their Regulatory expertise over other criteria. Look for a provider who has in-depth knowledge of regulations such as Good Manufacturing Practices (GMP), Good Clinical Practices (GCP), Good Distribution Practices (GDP), and relevant guidelines from Regulatory authorities like the United States Food and Drug Administration (USFDA) and the European Medicines Agency (EMA). Verify their experience in navigating Regulatory challenges specific to the pharmaceutical industry.
- Compliance with International Standards: Pharmaceutical companies often operate on a global scale, which necessitates compliance with international standards. Thus, ensure that the Regulatory pharma artwork service provider you choose has experience in handling international regulations and standards of different countries and regions. They should be well-versed in guidelines such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and have a track record of successful compliance across the globe.
- Quality Control Processes: Accuracy and precision are crucial in the pharmaceutical industry. Look for a Regulatory artwork service provider who has robust quality control processes in place. They should have stringent procedures for proofreading, error detection, and revision management. Inquire about their quality control measures, including how they handle version control, review cycles, and approval workflows. A provider with comprehensive quality control processes will help minimize errors and reduce the risk of Regulatory non-compliance.
- Technology and Automation Capabilities: Efficiency and speed are of paramount importance when it comes to managing artwork in the pharmaceutical industry. Evaluate the technology and automation capabilities of the Regulatory pharma artwork service provider. Look for providers who use advanced software and tools that are specifically designed for pharma artwork management, such as Computer-aided Design (CAD) software, Content Management Systems (CMS), and automated proofreading solutions. These technologies can streamline the artwork review and approval process, ensuring faster Turn Around Times (TATs) and minimizing human errors.
- Flexibility and Scalability: As a pharmaceutical company, your artwork requirements may vary in terms of volume and complexity. Choose a Regulatory artwork service provider who offers the flexibility and scalability to accommodate your changing needs. They should be able to handle both small-scale and large-scale projects as well as adapt their resources and processes accordingly. Ask about their capacity to handle peak periods, meet tight deadlines, and the availability of dedicated resources.
- Strong Communication and Collaboration: Effective communication and collaboration are vital when working with a Regulatory pharma artwork service provider. Ensure that the provider has a dedicated team of project managers, designers, and Regulatory experts who can effectively communicate and collaborate with your internal stakeholders. Look for providers who will be able to prioritize clear and timely communication, provide regular project updates, and are receptive to feedback and suggestions.
- Track Record and Reputation: Research the provider’s track record and reputation within the pharmaceutical industry. Look for testimonials, case studies, and client references to gain insights into their past performances. A reputable provider will have a proven track record of delivering high-quality Regulatory artwork services and maintaining strong client relationships.
- Knowledge of Artwork Regulations and Guidelines: The provider should deeply understand artwork guidelines and regulations specific to the pharmaceutical industry. They should stay up to date with the latest Regulatory changes and ensure that their processes align with the industry’s best practices. Inquire about their familiarity with specific requirements such as barcoding, serialization, tamper-evident packaging, and other industry-specific labeling standards.
- Data Security and Confidentiality: Pharmaceutical companies handle sensitive information, including proprietary product formulations, clinical trial data, and patient information. Ensure that the Regulatory artwork service provider has robust data security measures in place to protect your confidential information. Ask about their data encryption protocols, secure file transfer methods, and their adherence to data privacy regulations, for instance, whether they follow the General Data Protection Regulation (GDPR).
- Localization and Multilingual Capabilities: If you wish to market your pharmaceutical products globally, consider the provider’s localization and multilingual capabilities. They should be able to adapt artwork and labeling to meet the language and cultural requirements of different markets across the globe. Inquire about their experience in managing artwork translations and their process for ensuring accuracy and consistency across multiple languages.
- Audit and Inspection Support: Pharmaceutical companies are subject to regular audits and inspections by Regulatory authorities. Find out about the provider’s ability to support these processes. They should have experience in preparing artwork-related documentation and assisting in Regulatory inspections. Additionally, ensure that they maintain exhaustive records and comprehensive document control practices to facilitate the audits.
- Cost and Pricing Structure: Evaluate the cost and pricing structure offered by the Regulatory pharma artwork service provider. Request them for detailed pricing information, including any additional charges for rush orders or revisions. Consider the value they provide in terms of expertise, efficiency, and compliance. While cost is a key factor, also pay attention to quality, accuracy, and Regulatory compliance to avoid potential delays or penalties that may arise from non-compliant artwork.
Conclusion
Selecting the right Regulatory artwork service provider is paramount for pharmaceutical companies to achieve Regulatory compliance, streamline processes, and maintain product safety. By considering factors such as Regulatory expertise, international compliance, quality control processes, technology capabilities, flexibility, and collaboration, you can make an informed decision that aligns with your specific requirements.
Remember, choosing the right provider will help you meet the Regulatory requirements and contribute to your overall operational efficiency and success in the pharmaceutical industry. Freyr has been the preferred partner for multiple life sciences companies, providing them with end-to-end artwork services. Know more about our capabilities, make us your partner of choice, and stay compliant!