Advanced Therapy Medicinal Products (ATMPs) represent a cutting-edge approach to medicine, harnessing the power of cells, tissues, or genes to treat a variety of ailments. Due to their complexity and novelty, regulatory requirements for Advanced Therapy Medicinal Products (ATMPs) are understandably stringent. This extends to the artwork included in regulatory submissions, which plays a crucial role in conveying critical information clearly and concisely.
When adapting regulatory artwork for Advanced Therapy Medicinal Products (ATMPs), the following considerations should be considered:
Consult Our Regulatory Artwork Experts
Request a Consultation
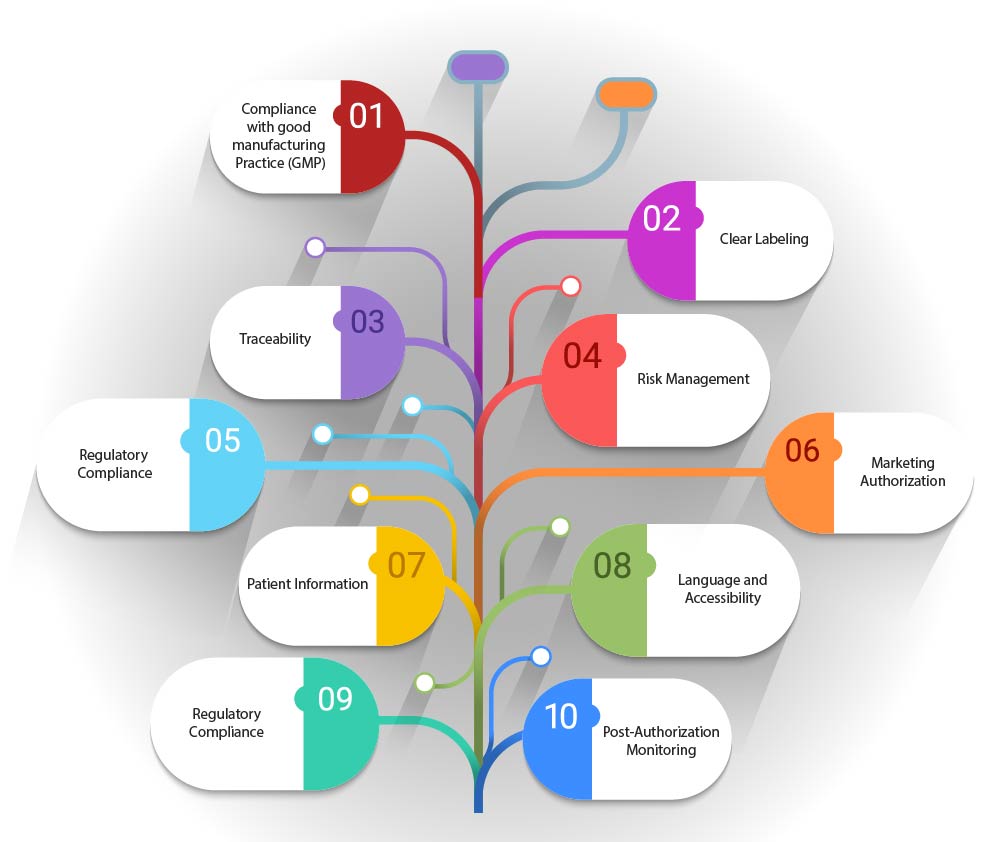
- Compliance with Good Manufacturing Practice (GMP): The artwork must comply with GMP guidelines specific to ATMPs, which may include details on the manufacturing process, quality control, and traceability.
- Clear Labeling: The artwork should indicate the nature of the product, including whether it is a gene therapy, somatic cell therapy, or tissue-engineered product. It should also include any specific storage or handling requirements.
- Traceability: Given the personalized nature of many Advanced Therapy Medicinal Products (ATMPs) the artwork should facilitate traceability. This may involve unique identifiers or barcodes that enable tracking of the product from manufacture to administration to the patient.
- Risk Management: Artwork should include information related to risk management, such as potential side effects or contraindications, to ensure that patients and healthcare providers are fully informed.
- Regulatory Compliance: The artwork must adhere to the regulatory framework established by legislation such as Regulation (EC) No 1394/2007, which sets out the requirements for marketing authorization, supervision, and pharmacovigilance of ATMPs.
- Marketing Authorization: The artwork should reflect the status of the product's marketing authorization, whether it is fully authorized, under clinical trial, or subject to hospital exemption.
- Patient Information: Artwork should include patient information leaflets that provide comprehensive information about the ATMP, its use, and any monitoring that may be required post-administration.
- Language and Accessibility: The artwork should be designed to be accessible and understandable, with consideration given to the language and legibility of the information provided.
- Regulatory Body Information: The artwork should include information about the relevant regulatory bodies, such as the European Medicines Agency (EMA) and the Committee for Advanced Therapies (CAT), which are responsible for the assessment and approval of Advanced Therapy Medicinal Products (ATMPs).
- Post-Authorization Monitoring: If applicable, the artwork should mention any post-authorization monitoring or additional studies that are required further to assess the long-term safety and efficacy of the ATMP.
Adapting regulatory artwork for ATMPs demands a meticulous approach, balancing scientific accuracy, clear communication, and adherence to evolving regulatory requirements. While internal expertise is valuable, navigating this intricate landscape can be immensely beneficial.
This is where partnering with a specialist like Freyr Solutions comes in. Freyr offers a team of regulatory artwork specialists with deep experience in ATMPs. They understand the specific challenges associated with ATMP artwork and possess the technical skills to create clear, compliant, and impactful visuals.
In today's competitive landscape, a strong regulatory submission can be the difference between success and failure. By partnering with Freyr Solutions, you ensure your ATMP artwork effectively showcases the potential of your therapy, paving the way for a smoother regulatory journey. With their specialized knowledge and collaborative approach, Freyr becomes an invaluable asset in bringing your groundbreaking ATMP to market. Contact us Today!