Over the years, with the advancements of software and digitalization, there has been a seismic shift in how medical devices are administered and delivered. The integration of software with medical devices has rapidly increased and driving incredible advancements in delivering healthcare solutions across various domains like diagnosis, disease prevention and treatment of an injury or illness.
However, the effect of software on the safety and performance of medical devices has been dubious, particularly, when the device itself is a software alone product. Hence, the medical device software regulations are constantly revised to determine the consideration of software as a medical device (SaMD). Recently, the advisory board of the European Commission - Medical Device Coordination Group (MDCG) has focused on improving the regulations of medical device software and published a guidance describing the approach to be applied, while determining whether a software is a medical device or not. What does the guidance outline? Let us unveil.
The Scope of the Guidance
The MDCG guidance covers both the medical device software and in vitro diagnostic (IVD) medical device software. As per the document, a Medical Device Software (MDSW) is defined as a software that is intended to be used alone or in combination, for a purpose as specified in the definition of a “medical device” in the Medical Devices Regulation 2017/745 (MDR) or In Vitro Diagnostic Medical Devices Regulation 2017/746 (IVDR). It outlines the criteria to be applied for determining whether a software that is subject to review is a medical device or not and intends to provide additional clarifications and recommendations on MDSW for medical device manufacturers and other parties.
Firstly, the guidance sets out the most important terms used in the context of MDSW, which include:
Intended purpose: The use for which a device is intended according to the data supplied by the manufacturer on the label, in the instructions for use, or promotional or sales materials or statements and as specified by the manufacturer in the clinical evaluation.
Accessory: An article which, whilst not being itself a medical device, is intended by its manufacturer to be used together with one or several medical device(s) to specifically enable the medical device(s) to be used in accordance with its/their intended purpose(s) or to assist the medical device functionality of the medical device(s) specifically and directly in terms of its/their intended purpose(s). Additionally, the MDCG mentions that the software accessory may be driving or influencing the use of a medical device and the instructions for use and other documentation provided by the manufacturer should contain details about the way the appropriate software and accessories should be selected.
Software: It stands for a set of instructions that processes input data and creates output data.
Medical Device Software Determination
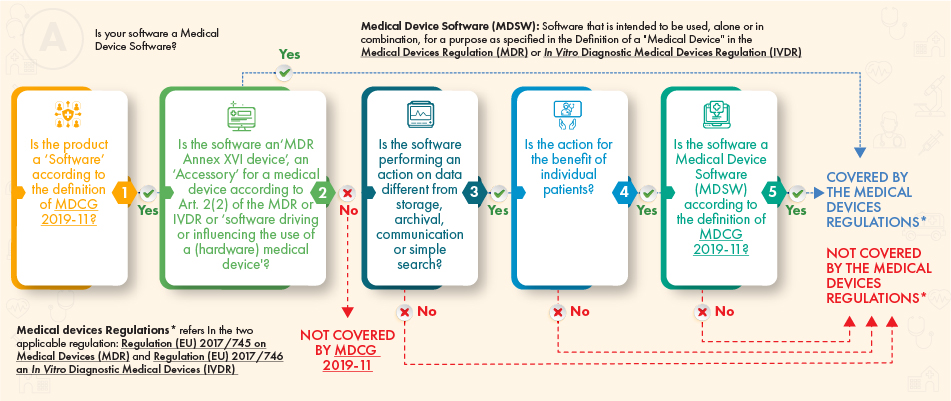
As per the above guidance flowchart, the software in question should be subject to regulation, if it meets the following criteria:
- The definition of a medical device, an accessory thereto, or drives the operations of the medical device, or
- It performs additional processing of data (not only storage or communication) and its action creates benefits for the patients and meets the definition of medical device software in accordance with the MDCG guidance
In Vitro Diagnostic Medical Devices Software Determination
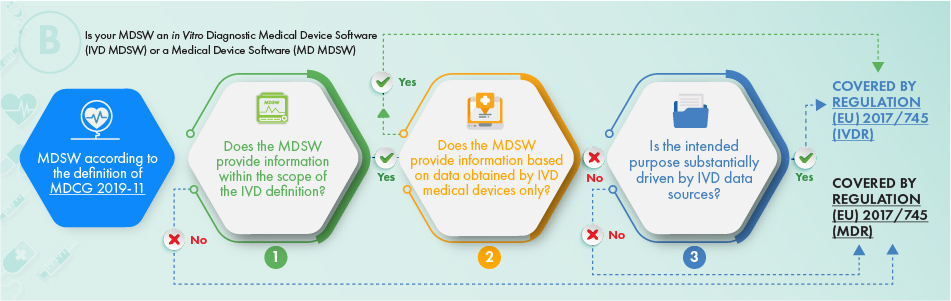
The above flowchart describes the approach to be applied, with regard to the products intended for in vitro diagnostic purposes. To ascertain whether the software in question should be subject to regulation, the following criteria should be addressed:
- The definition of a medical device, an accessory thereto, or drives the operations of the medical device, or
- It provides the information usually provided by in vitro diagnostic medical devices and only the information collected from an in vitro diagnostic medical device, or
- The intended purpose of the software is related to IVDR matters
As per the MDCG guidance, the type of interconnection between the medical device software and the device does not affect the qualification of the software as a device under the MDR and IVDR. A medical device software could exist either as a stand-alone product or be incorporated in a hardware device and clarifies the following Regulatory requirements:
- Considering its qualification and classification, a stand-alone medical device software product must be subjected to full scope of Regulatory procedures in accordance with the applicable legislation.
- A medical device software that is an integral component or part of a hardware medical device could be placed on the market under the simplified procedure. It would be subject to review not separately, but during the general assessment of the hardware medical device itself.
To summarize, the MDCG guidance covers the vital aspects related to the classification of medical device software and the determination of the Regulatory requirements to be applied. Medical device manufacturers, software developers and other parties must follow and implement the MDCG recommendations to ensure compliance. To gain further insights on determining your software as a medical device, consult a Regulatory expert. Stay informed. Stay compliant.









