
In the highly regulated landscape of the Life Sciences industry, adhering to Product-specific Guidelines (PSGs) is paramount for successful drug development and approval. PSGs are comprehensive documents issued by global Health Authorities (HAs) to provide guidance for pharmaceutical product approvals within specific therapeutic categories.
This blog details the purpose, relevance, and the HA’s perspective on issuing PSGs. Understanding the rationale and expectations laid out in PSGs that enable the strategic success of life sciences companies is detailed in this artifact.
Understanding Product-specific Guidelines and Their Types
PSGs play a pivotal role in shaping the Regulatory pathway for pharmaceutical products. By providing targeted guidance, HAs ensure consistency and robust evaluations of product applications within specific therapeutic categories. By adhering to PSGs, pharma companies can navigate the intricate Regulatory landscape, ensuring their products meet the required safety and efficacy standards.
PSGs are not limited to generic drug development. They also cover a wide range of pharmaceutical products, such as the following:
- For Innovator drugs: PSGs provide specific guidance on preclinical studies, clinical trial design, safety assessment, pharmacokinetics, and efficacy endpoints. They guide companies on the type and amount of data required to demonstrate the safety and efficacy of their innovative drugs.
- For Biosimilars: PSGs outline data requirements, analytical methods, and clinical study-design considerations for biosimilar products. By following them, biosimilar companies can demonstrate the similarity of their products to the reference biologic, paving the way for approval and market access.
- For Biologics: These guidelines address critical aspects, such as manufacturing, characterization, and comparability studies, ensuring that the biologics meet the approved standards.
Development of Product-Specific Guidelines & Processes
Developing PSGs is a collaborative effort involving HAs, scientific experts, and industry stakeholders. The following are the PSG's development stages:
Stage 1 - Comprehensive assessment of the therapeutic area: HAs identify gaps in existing guidelines and initiate the development of PSGs to address those gaps. Scientific experts and industry stakeholders are then engaged to contribute their expertise, ensuring the draft guidelines are evidence-based and practical to implement.
Stage 2 - Public Consultation: Draft PSGs are now available for public review and feedback. This open and transparent approach allows stakeholders, including Healthcare Professionals (HCPs), patient groups, and industry representatives, to provide valuable input and improve the overall quality and relevance of the guidelines.
Stage 3:Internal Review Stage: While the draft PSGs are in public consultation, the PSG development team within the HAs undertakes a rigorous internal review, ensuring that the guidelines meet the necessary Regulatory requirements and align with the broader Regulatory framework.
Stage 4 - Finalization: Once finalized, PSGs are published and made accessible to the industry, providing clear and actionable guidance for product development and submission.
Components of PSGs
Life sciences companies must have a clear understanding of the PSGs’ components. The following pie chart illustrates them in detail:
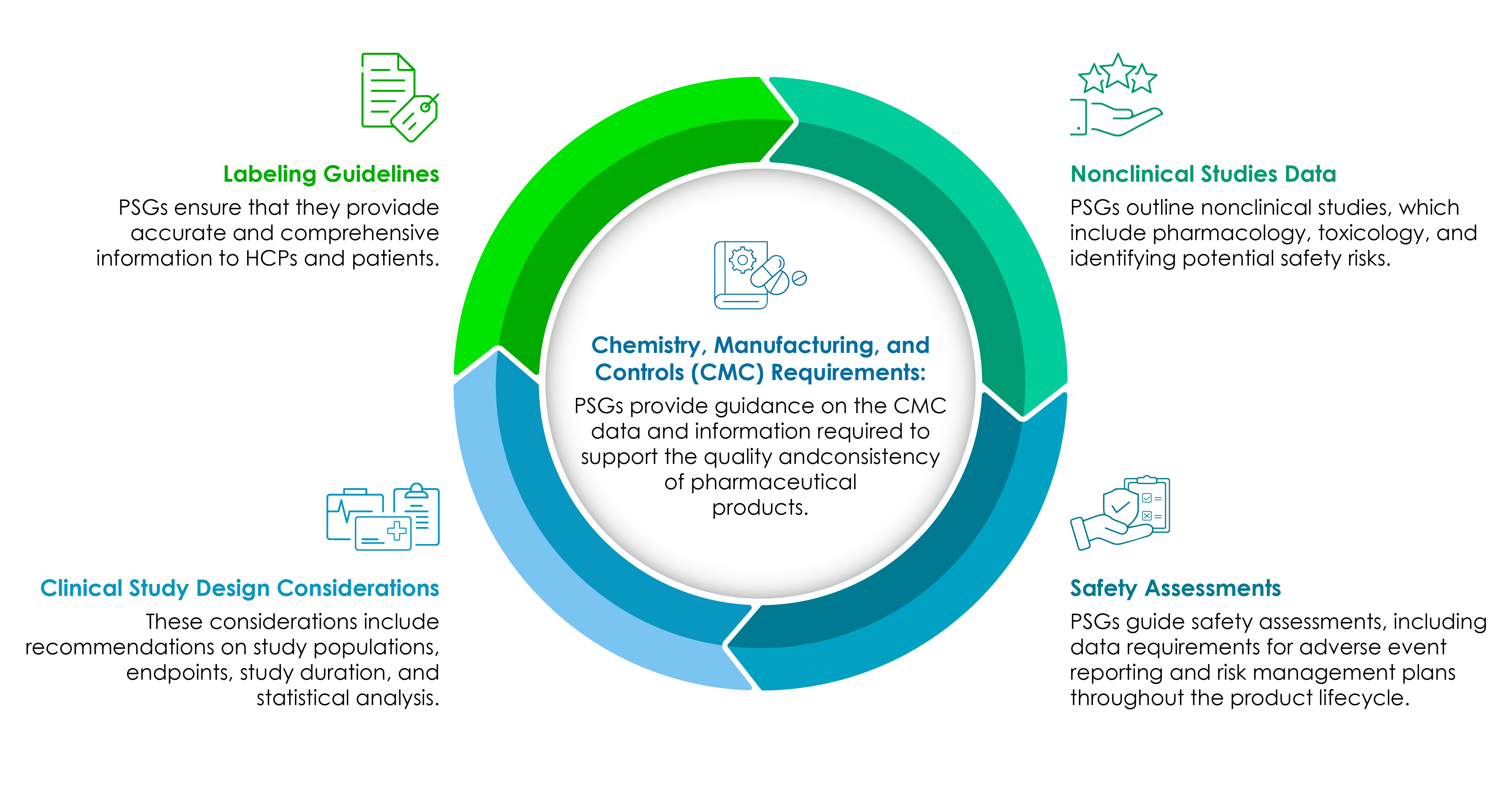
CMC remains the core of every Regulatory-focused pharmaceutical product development.
Navigating PSGs
Navigating PSGs in a Regulatory-compliant manner can be achieved by the following enlisted points/strategies:
- Early Engagement with Health Authorities: Such engagement fosters a collaborative approach and helps address potential challenges proactively.
- Scientific Expertise: Leveraging expert inputs ensure that study designs, analytical methods, and safety assessments align with the highest scientific standards.
- Regulatory Consultants: Consultants provide expert guidance, ensuring the development plans and Regulatory submissions are successful.
- Comprehensive PSG Review: Companies should identify specific PSG sections applicable to their product and align their development plans accordingly.
- Continuous Monitoring: PSGs are dynamic documents that may undergo updates or revisions frequently. Companies must monitor the changes in PSGs to ensure Regulatory compliance with the latest PSG requirements.
Expert Regulatory guidance makes PSG navigation easy. Consult now!
Conclusion
Product-specific Guidelines (PSGs) form the backbone of Regulatory submissions for pharmaceutical products within specific therapeutic categories. Understanding the HA’s perspectives behind PSG issuance is critical for life sciences companies seeking to expedite approvals, ensure product quality, and navigate the global Regulatory landscape seamlessly.
As a leading Regulatory services provider, Freyr recognizes the significance of adhering to best practices and leveraging expertise that will guide life sciences companies to align their product development strategies per the PSG requirements. Contact us today to learn more about our services and how we can help you achieve your goals.









