Introduction
The rise of biopharmaceuticals has been nothing short of a revolution in the life sciences arena. These complex, large-molecule drugs have transformed the healthcare landscape, offering targeted and effective treatments for an array of diseases. As biopharmaceuticals continue to surge, the United States Food and Drug Administration (US FDA) plays a pivotal role in ensuring their safety and efficacy.
In this blog, we delve into the remarkable ascent of biopharmaceuticals and gain insights into the US FDA's perspective on this transformative field. Additionally, we understand the crucial role of Regulatory vendors in navigating the complexities of biopharmaceutical regulation.
Biopharmaceuticals Revolution
Due to the surge in research and development, the biopharmaceutical industry has witnessed remarkable growth. Unlike traditional small-molecule drugs, biopharmaceuticals are derived from living organisms or biological processes, including proteins, nucleic acids, and cells. These large, complex molecules offer several advantages, such as high specificity and reduced side effects, making them ideal for treating a variety of diseases, from cancer to autoimmune disorders.
FDA's Evolving Role
As the biopharmaceutical industry flourishes, the US FDA has adapted its Regulatory approach to accommodate these innovative therapies. The agency's perspective reflects a commitment to facilitating innovation while maintaining the highest standards of patient safety.
- Balancing Innovation and Safety:
One of the primary challenges the FDA faces in regulating biopharmaceuticals is finding the delicate balance between encouraging innovation and safeguarding patient welfare. Biopharmaceutical development can be lengthy and costly, and Regulatory processes must be agile to keep pace with scientific advancements.
- Regulatory Pathways for Biopharmaceuticals:
The FDA provides various Regulatory pathways tailored to the unique characteristics of biopharmaceuticals. This includes pathways for biosimilars, which are similar but not identical to already-approved biopharmaceuticals, and expedited review programs for breakthrough therapies that show substantial promise in treating serious diseases.
- Ensuring Quality and Efficacy:
The FDA's perspective on biopharmaceuticals extends to ensuring the quality and efficacy of these treatments. Biopharmaceutical manufacturing is highly complex and demands stringent quality control. The FDA collaborates closely with manufacturers to establish and uphold high standards, including robust processes and controls to minimize variations in drug products.
- Safety Monitoring and Post-Market Surveillance:
Beyond the initial approval, the FDA's role in monitoring the safety and efficacy of biopharmaceuticals continues post-market. This includes adverse event reporting, inspections, and ongoing safety assessments to identify and address potential risks. The agency's perspective is firmly rooted in the commitment to ensuring that the benefits of biopharmaceuticals continue to outweigh any risks.
- Upholding patient-centric approaches:
The US FDA's perspective is in alignment with the industry's trend toward patient-centric healthcare. It encourages the involvement of patient communities and stakeholders in the Regulatory process, ensuring that the patient's voice is heard, and their needs are considered in the evaluation of biopharmaceuticals.
- Fostering global collaboration:
The rise of biopharmaceuticals has transcended national borders. The FDA actively collaborates with international Regulatory agencies to establish harmonized guidelines for the biopharmaceutical development and approval of these therapies. Such collaborations help streamline the global development of biopharmaceuticals, making these innovative treatments accessible to a broader patient population.
Role of Regulatory Vendors
Regulatory vendors can act as strategic partners in the era of biopharmaceuticals by helping pharmaceutical companies navigate the intricate landscape:
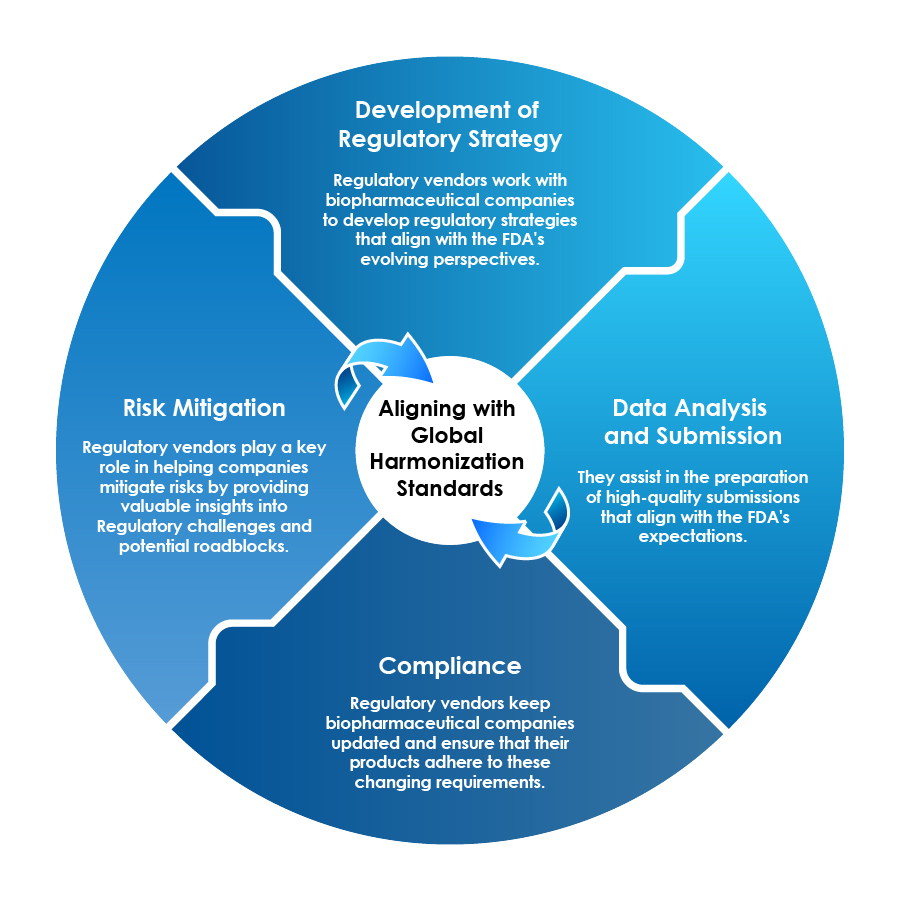
Regulatory Vendors Ensure Alignment with Global Standards
Conclusion
The ascent of biopharmaceuticals represents a monumental shift in healthcare, offering targeted and effective treatments for diverse diseases. FDA's perspective shows continuous adaptation and innovation in Regulatory approaches toward biopharmaceutical development. Moreover, Regulatory vendors, acting as crucial partners, ensure that biopharmaceutical companies successfully navigate the intricate landscape, facilitating the journey from innovation to market availability.
The synergy between the FDA and Regulatory vendors empowers the industry to meet the evolving demands of patient care and drive forward a future of groundbreaking biopharmaceutical innovations. Our experts at Freyr are well-versed in handling such Regulatory imperatives, contact us to gain more insights.