
In India, a medical device test license, issued by the Central Drugs Standard Control Organization (CDSCO), is a temporary license that allows a manufacturer to conduct tests on their medical device before they can obtain a permanent license for it. The test license allows the manufacturer to conduct clinical trials and other tests on the device and gather data on its safety and efficacy. The CDSCO requires the manufacturer to obtain this license to ensure that the device is safe and effective for patients’ use.
Importance of the Test License
A test license is key to ensuring the efficacy, safety, and quality expectations of a device, as per medical device regulations in India. Medical devices can have a substantial impact on patients’ health outcomes, as they are used to diagnose, treat, and alleviate infections and medical conditions. Thus, it is essential to have a Regulatory framework in place to guarantee that medical devices adhere to a set of standards before they are marketed.
The test license requirement is an important part of the Regulatory process in India, and it ensures that medical devices are thoroughly tested before being commercialized. Without this restriction, manufacturers would be able to potentially market devices that are not safe or effective, which in turn could have serious consequences. The CDSCO assists manufacturers in obtaining the test license by helping them adhere to the Regulatory standards and ensuring that medical devices are safe and secure for human use.
Who Can Apply for the Test License?
A person who intends to manufacture or import medical devices for the purpose of clinical investigations, tests, evaluations, demonstrations, or training is eligible to apply for the test license by filling out a form in an online portal.
Figure 1: The Test License Application Process
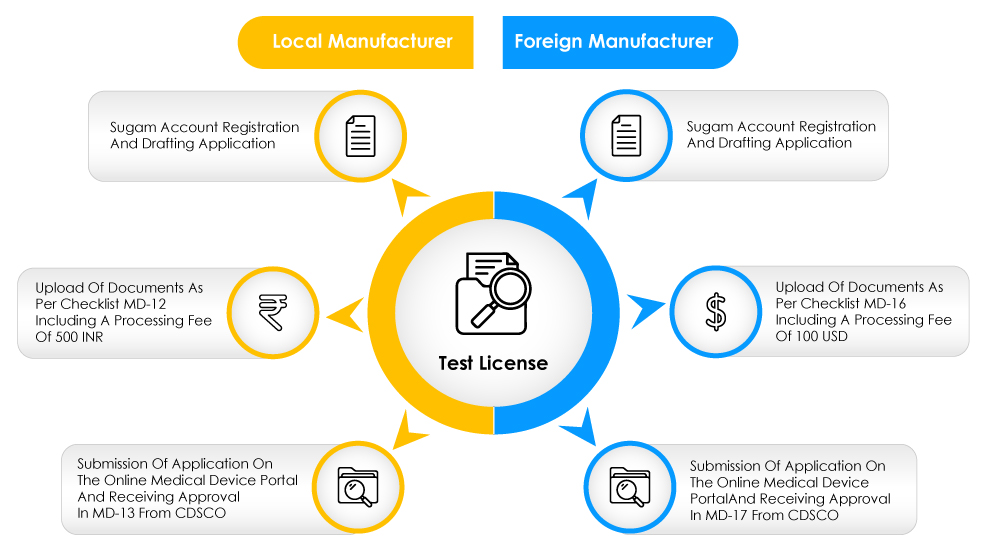
Requirements for Obtaining the Test License
The regulations for obtaining a medical device test license are quite stringent. The manufacturer must provide the following detailed information on their device and its performance to obtain the license:
- Device Description: They need to provide a detailed description of the device that covers aspects like its design, specifications, and intended use.
- Manufacturing Process: They must provide information on the manufacturing process of the device, including any quality control measures that might be in place to ensure its safety and efficacy.
- A Testing Plan: They need to submit a detailed testing plan outlining the specific tests that will be performed on the device, the number of patients that will be enrolled in the study, and the duration of the study.
- Clinical Data: They must provide clinical data on the safety and performance of the device, which includes information on any adverse events that might have occurred during the clinical trials, as well as data on the efficacy and performance of the device.
- Risk Analysis: They need to conduct a risk analysis of the device, identifying potential risks associated with its use and outlining strategies for mitigating those risks.
- Labeling and Instructions for Use (IFU): They should provide labeling and IFU of the device, including any warnings or precautions that users need to be aware of.
The Process for Obtaining the Test License
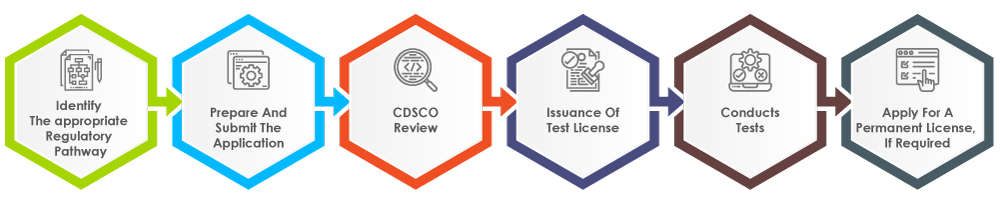
The process for obtaining a medical device test license in India involves several steps, as outlined below in Figure 2.
Figure 2: The Process for Obtaining a Medical Device Test License
The Duration of the Test License
A medical device test license is typically valid for three (03) years. With respect to the specific device and the testing plan described in the application, the time frame may vary. When the license expires, the manufacturer must request for a fresh license or a permanent license to continue marketing their device in India. However, in some cases, manufacturers may need more time to carry out the necessary testing. Under these circumstances, they can ask for an extension of the license. However, they need to apply for the extension before the license expires and is subject to review by the CDSCO.The medical device test license is an important step in the Regulatory framework of medical devices in India. To safeguard patients’ safety and promote public health, the license ensures that the devices are adequately tested and assessed before they are marketed to the public. Manufacturers must provide all the required information and follow all the pertinent rules to receive the license.
To know more about a medical device test license, reach out to our Regulatory expert. Stay informed! Stay compliant!









