AI is the new buzzword, and it does not seem like its buzz is getting any fainter any time soon. We all know that Artificial Intelligence (AI) and Machine Learning (ML) have been a transformative force across various industries, and the medical sector is no exception. In recent years, AI/ML has made significant strides in revolutionizing Regulatory operations within the medical industry. Regulatory authorities worldwide are embracing the potential of AI/ML to streamline processes, enhance decision-making, and improve overall healthcare quality. Let us explore how AI/ML is poised to change Regulatory operations in the medical field and what are the Regulatory Authorities' guidelines for this technological advancement.
The Role of AI/ML in Regulatory Operations
Artificial intelligence offers a promising solution to the Regulatory challenges. Here's how AI/ML is poised to transform Regulatory operations in the medical industry:
- Informed decision-making with data Analysis and predictive modeling. AI algorithms can process vast datasets rapidly, enabling Regulatory authorities to identify trends, potential risks, and anomalies more effectively. Predictive modeling can help assess the safety and efficacy of drugs with greater accuracy by avoiding manual errors.
- Automation of routine tasks enables regulatory authorities to focus on more strategic tasks. AI-powered automation can streamline routine Regulatory tasks such as data entry, document review, and compliance monitoring, reducing the risk of errors and speeding up processes.
- Enhanced compliance monitoring ensures that regulatory authorities are up to date. AI can continuously monitor data for compliance with regulations and standards, flagging deviations or potential issues in real-time, and reducing the risk of product recalls or Regulatory violations.
- Drug discovery and development ensures that regulatory authorities are able to bring lifesaving drugs faster to market with a more economical approach. AI-driven drug discovery can significantly accelerate the development process, potentially leading to faster approvals and reduced development costs. ML algorithms can analyze biological data, identify potential drug candidates, and predict their efficacy, significantly reducing the time and cost associated with bringing new drugs to market.
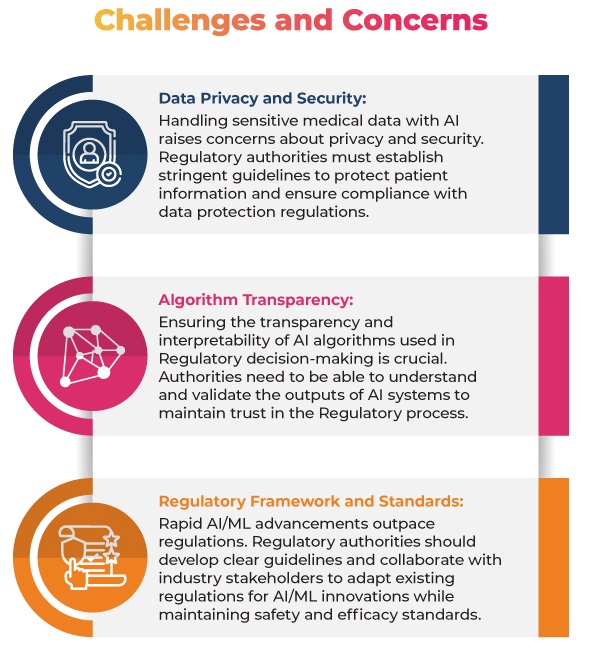
Regulatory Authority Guidelines
To address the concerns, the Regulatory Authorities have already issued some guidelines and frameworks for using AI/ML in the medical field:
FDA: Guidelines on AI/ML in Medical Devices: The FDA issued guidelines that outline the Regulatory framework for AI and ML-based Software as a Medical Device (SaMD). These guidelines provide recommendations for developing, evaluating, and validating SaMDs, including using real-world performance monitoring, algorithm change protocols, and quality system practices. The aim is to ensure the safety and effectiveness of AI/ML-based medical devices while promoting innovation and development in this critical area.
EMA: The use of Artificial Intelligence (AI) in the medicinal product lifecycle: This reflection paper addresses the use of AI/ML in the entire lifecycle of medicinal products, emphasizing safety and efficacy. It aims to provide Regulatory guidance in the rapidly evolving AI/ML landscape, focusing on the scientific principles necessary for evaluating these technologies in drug development, authorization, and post-authorization phases.
MHRA: Guidance on Software and AI as a Medical Device: The Medicines and Healthcare products Regulatory Agency (MHRA) in the UK published guidance on AI/ML in medical software. It outlines Regulatory expectations for AI/ML-based SaMD, emphasizing the importance of data quality and risk management.
The advent of AI/ML in the medical industry is transforming Regulatory operations, making them more efficient, data-driven, and adaptive. It has the potential to streamline processes, reduce compliance costs, accelerate drug development, and ultimately enhance public health outcomes. However, this transformative shift must be executed carefully, with a focus on validation, ethics, and collaboration to gain the trust of Regulatory authorities. As AI/ML continues to evolve, it will be fascinating to witness the ongoing transformation of pharmaceutical Regulatory operations and its positive impact on patient safety and innovation in the field.
Reach out to hello@freyrdigital.com to discover our AI/ML-driven innovations streamlining regulatory operations in the medical industry. Contact us today.