SmPC or Summary of Product Characteristics is a legal document which is a part of the marketing authorization of every medicine. The document acts as a basis of information on the use of medicines for healthcare professionals. The information included in the SmPC is updated regularly as per the emergence of the latest information. SmPC contains more information than a Package Leaflet. The SmPC information can be found through the following sources:
- Websites of Health Authorities; such as the European Medicines Agency (EMA)
- Medicine dictionaries
What consists of an SmPC?
- Information related to the medicine usage
- Qualitative and quantitative information on medicines’ benefits and risks
- Dosage information
- Administration method
- Pharmacological information
- Individual care information
Structure of an SmPC
The structure of the SmPC is defined by the European pharmaceutical legislation. The information included in the SmPC should be product specific and can be cross-referenced to avoid any redundancy. It should be documented in a clear language and should not lead to any ambiguity. The SmPC is divided into 6 major sections:
- Name of the product
- Composition
- Pharmaceutical Form
- Clinical particulars – Includes therapeutic indications, recommendation for dosages and safety information
- Pharmacological properties – Takes into account the therapeutic indications of the clinical elements and their potential adverse drug reactions
- Pharmaceutical particulars – Includes Regulatory information related to the drug
As per the EMA structure of an SmPC can be depicted as:
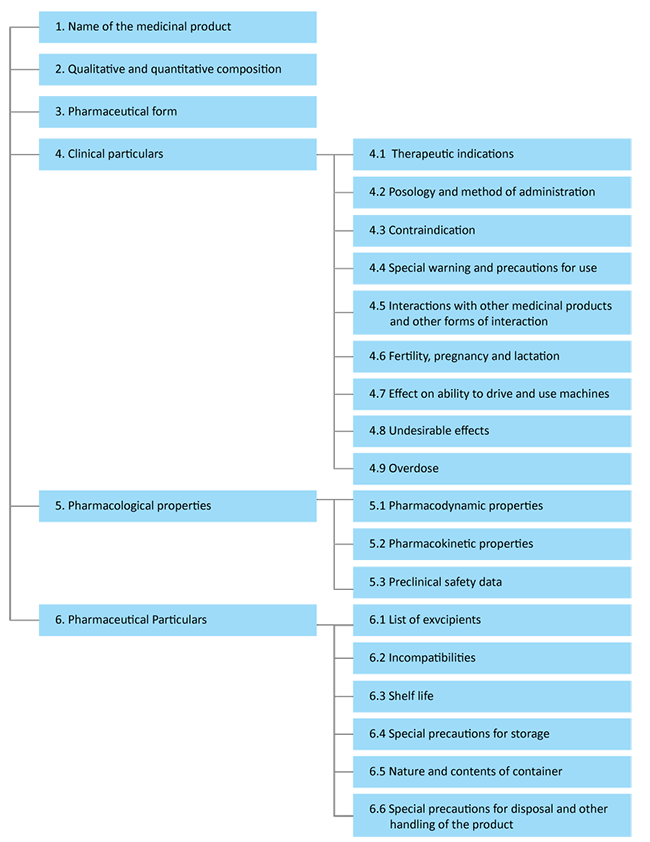
Reference: EMA
What Information is Excluded from an SmPC?
- Information available in the public assessment reports (scientific development details)
- Unapproved indication information
- Issues which lack data
- General advice on pharmacological conditions
Maintaining an SmPC is important for the life cycle of any medicine as it is a part of its marketing authorization. Therefore, authoring a compliant SmPC is highly recommended. Are you looking for expert Regulatory assistance to develop an SmPC? Reach out to Freyr @ sales@freyrsolutions.com.