Shonin (Pre-Market Approval) is the Regulatory pathway for registering medical devices in Japan. Shonin pathway is primarily for registration of Class II and III medical devices for which the PMDA’s classification standards are not available. For the high-risk Class IV devices also, manufacturers should submit the Shonin application. PMDA is responsible for the review and approval of the Shonin application.
What are the Other Device Registration Pathways in Japan?
In addition to Shonin, the Todokede and Ninsho pathways are also used for medical device approvals in Japan. Medical device manufacturers can choose one of them depending on the risk class of the device and the availability of predicates in Japan. The manufacturer shall identify the device classification and research for the availability of the Japanese Industrial Standard (JIS) before determining the applicable registration pathway.
- Todokede (Pre-Market Submission) – It is applicable to Class I devices and requires manufacturers to submit a pre-market notification to the PMDA for approval.
- Ninsho (Pre-Market Certification) - It is applicable to Class II and III generic devices having certification standards (JIS Standards). The registered Certification Body (RCB) is responsible for the review and approval of the application.
What are the Prerequisites for Shonin Registration?
Manufacturers registering their devices through the Shonin pathway must meticulously plan the submissions. They must ensure the following:
- Submitting general device data, such as medical device category, intended use, efficacy risk analysis data, clinical data, etc.
- Providing Summary of Technical Documentation (STED)
- Provision of documents in Japanese language only
- The foreign manufacturers mandatorily must appoint a Marketing Authorization Holder (MAH) or a Designated Marketing Authorization Holder (DMAH)
- Foreign manufacturers must obtain Foreign Manufacturer Registration (FMR) certificate for their manufacturing establishments.
What are the QMS Requirements for Device Registration under the Shonin pathway?
Manufacturers must comply with all the QMS requirements defined under Ordinance 169. The sponsor or DMAH or MAH must apply to the PMDA. The PMDA carries out a detailed QMS inspection of the manufacturer’s facility and issues the certificate upon satisfactory implementation of the QMS.
What is the Registration process for device approval under Shonin pathway?
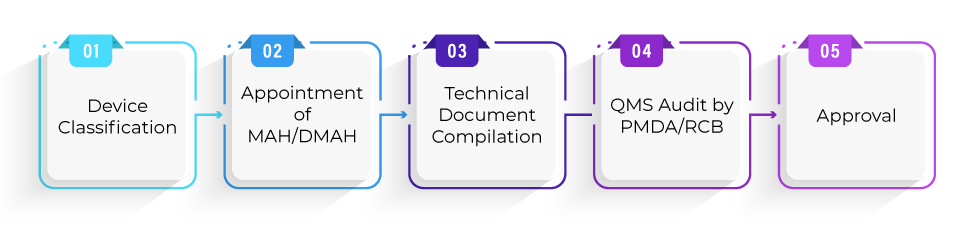
What is the Average timeline required for device approval under the Shonin pathway?
The PMDA usually requires 12 months for technical assessment from the date of receipt of the Shonin application. The manufacturer must consider the time needed to prepare the submission documents or conduct clinical studies in their project timelines.
Is there any Expiration Timeline for device registration under Shonin pathway?
Medical device registration doesn’t expire, but the sponsor should renew the QMS certificates every five (05) years.
Japan is a lucrative market but intrinsically comes with Regulatory complexities and linguistic barriers. The manufacturers must consider these factors and proactively plan their go-to-market (GTM) strategy for Japan. The medical device and IVD manufacturers may choose to outsource all the Regulatory nuances to a reliable Regulatory partner and utilize the resources to focus on other essential components.
To know more about Shonin medical device approval in Japan or any other PMDA Japan regulations, Contact Freyr’s Regulatory experts, today.