
Regulatory artwork compliance is of utmost importance in the pharmaceutical industry to ensure patient safety and adherence to Regulatory standards. Pharmaceutical companies must navigate complex Regulatory requirements and maintain compliance throughout the artwork management process. In this blog post, we will discuss ten best practices that pharmaceutical companies can implement to effectively navigate and sustain Regulatory artwork compliance.
Ten Best Practices to navigate and sustain Regulatory Artwork Compliance
Enhance Your Artwork Compliance Today!
Enhance Your Artwork Compliance Today
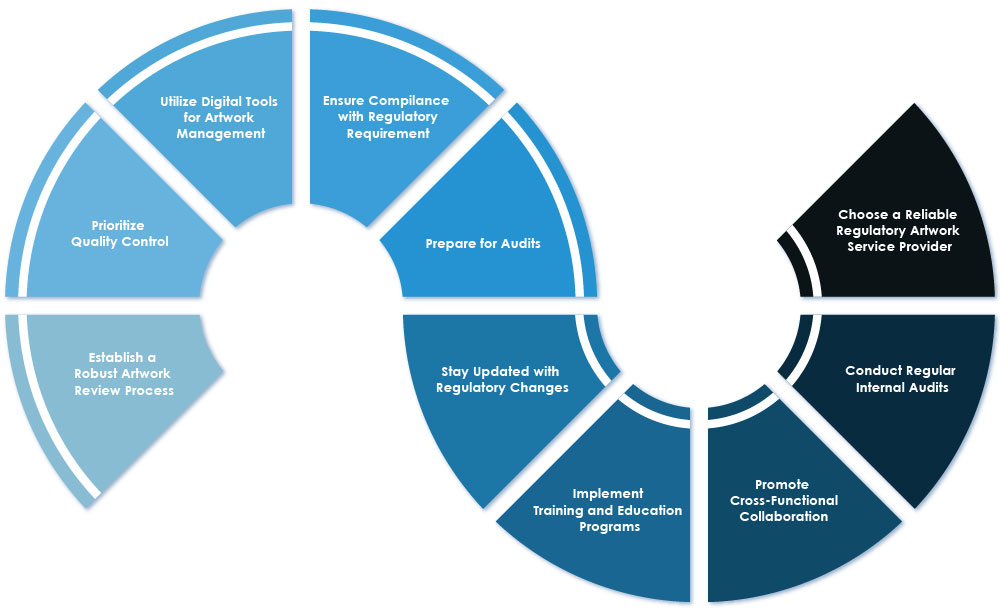
1. Establish a Robust Artwork Review Process: Develop a standardized artwork review process that includes clear procedures, checklists, and collaboration among stakeholders. This process ensures that artwork meets Regulatory requirements and contains all necessary information.
2. Prioritize Quality Control: Emphasize the significance of quality control in Regulatory artwork services. Implement rigorous quality control measures to ensure accuracy, consistency, and compliance with Regulatory standards.
3. Utilize Digital Tools for Artwork Management: Leverage digital tools to streamline artwork management processes. These tools can automate workflows, reduce errors, and enhance collaboration among stakeholders, resulting in improved efficiency and compliance.
4. Ensure Compliance with Regulatory Requirements: Adhere to Regulatory requirements related to labeling and packaging. Ensure that all artwork and labels comply with Regulatory specifications, including the inclusion of necessary information and adherence to labeling guidelines.
5. Prepare for Audits: Maintain accurate records and documentation of artwork and labeling processes. Be prepared for audits by Regulatory authorities, ensuring that all processes are consistent with Regulatory requirements and that necessary information is readily available.
6. Stay Updated with Regulatory Changes: Stay informed about Regulatory changes and updates that may impact artwork compliance. Regularly monitor Regulatory guidelines and adapt artwork processes accordingly to ensure ongoing compliance.
7. Implement Training and Education Programs: Provide comprehensive training and education programs for employees involved in artwork management. Ensure that they are well-versed in Regulatory requirements, quality control measures, and the proper use of digital tools.
8. Promote Cross-Functional Collaboration: Foster collaboration among different departments, including Regulatory affairs, labeling, marketing, and sales. Encourage open communication and knowledge sharing to ensure alignment and compliance throughout the artwork process.
9. Conduct Regular Internal Audits: Perform regular internal audits to assess compliance with Regulatory artwork requirements. Identify any gaps or areas for improvement and take corrective actions to maintain and enhance compliance.
10. Choose a Reliable Regulatory Artwork Service Provider: Select a reputable Regulatory Artwork Service Provider who has experience and expertise in the pharmaceutical industry. Consider factors such as their track record, compliance with Regulatory standards, and ability to meet specific requirements.
Navigating and sustaining Regulatory artwork compliance in the pharmaceutical industry is crucial for patient safety and Regulatory adherence. By implementing these ten best practices pharmaceutical companies can effectively navigate and sustain Regulatory artwork compliance, ensuring the highest standards of patient safety and Regulatory compliance. Freyr is an experienced Regulatory artwork service provider with a team of experts with years of experience in Regulatory artwork compliance. Explore more about our expertise and contact us.









