
Clinical labeling is a critical component in bringing a pharmaceutical product to market. It ensures patient safety, compliance, and effective communication of essential information. In this blog, we will delve into the fundamentals of clinical labeling and explore why understanding its essentials is crucial for achieving Regulatory approval.
The Significance of Clinical Labeling
Clinical labeling goes beyond being a mere identifier on a medication package. It is a comprehensive system of information that encompasses everything from dosage instructions to potential side effects. Its primary purposes include:
- Patient Safety: Accurate and clear labeling is essential to prevent medication errors, ensuring patients receive the right treatment at the right time.
- Regulatory Compliance: Regulatory bodies, such as the FDA (Food and Drug Administration) or EMA (European Medicines Agency), have strict guidelines regarding the content and format of clinical labels. Adherence to these regulations is non-negotiable for market approval.
- Effective Communication: Healthcare professionals, patients, and Regulatory agencies rely on clinical labeling for clear and concise information. Proper communication is vital for the safe and effective use of the medication.
Optimize Your Clinical Labeling Strategy
Request a Consultation
Components of Clinical Labeling
Understanding the essential components of clinical labeling is crucial for creating a comprehensive and compliant label. These components include:
- Drug Name and Strength: Clearly stating the drug’s name and its strength is fundamental for proper identification.
- Dosage and Administration Instructions: Accurate dosage information, along with explicit administration instructions, helps healthcare professionals ensure patients use the medication correctly.
- Indications and Usage: Describing the conditions the drug is intended to treat provides context for its use.
- Contraindications and Warnings: Highlighting situations where the drug should not be used and providing warnings about potential risks are critical for patient safety.
- Side Effects and Adverse Reactions: Transparent information about possible side effects helps patients and healthcare providers make informed decisions.
- Storage and Handling Instructions: Proper storage and handling guidelines maintain the stability and efficacy of the medication.
- Expiration Date: Clearly indicating the expiration date ensures patients use the medication within its safe and effective period.
Regulatory Landscape
Navigating the Regulatory landscape is a complex task, as different regions may have unique requirements. However, common threads exist, such as the need for compliance with Good Manufacturing Practices (GMP) and submission of the drug label for approval.
- FDA Label Requirements: The FDA provides detailed guidance on prescription drug labeling requirements, covering everything from content to format and typography.
The following representation is a summary of the information to be included in human prescription drugs per the FDA’s regulations:
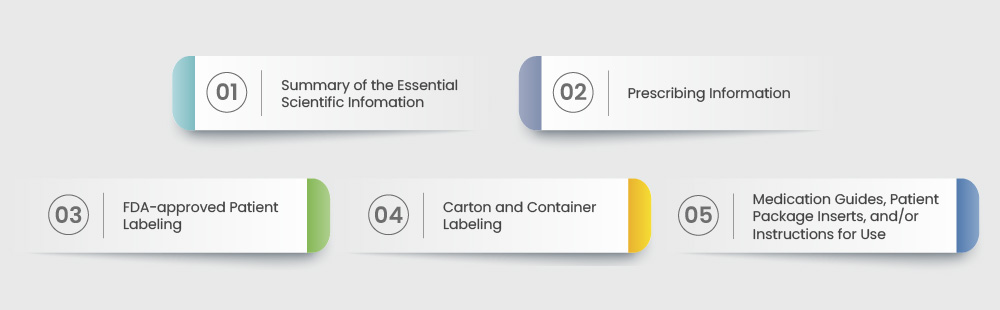
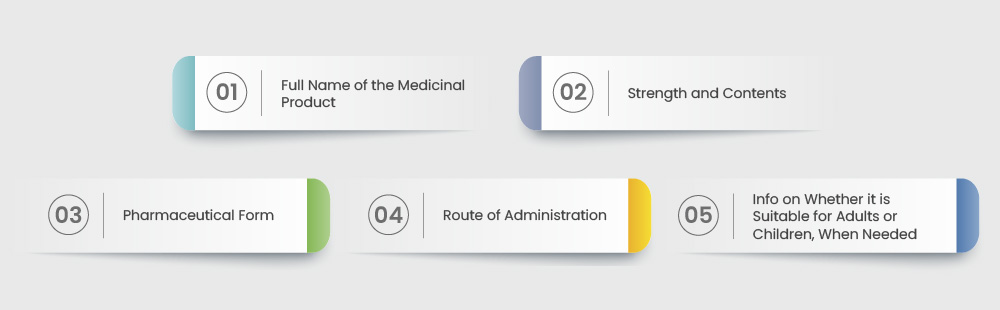
- EMA Guidelines: The EMA has its own guidelines that pharmaceutical companies must follow for market approval in Europe. Some of these are listed below:
Labeling Challenges and Solutions
Creating compliant clinical labels is not without its challenges. These may include frequent updates, translations for global markets, and changes in Regulatory requirements. Utilizing advanced technologies, such as label management software and automation, can streamline the process and reduce the risk of errors.
Conclusion
In drug development and Regulatory approval processes, clinical labeling stands as a linchpin between pharmaceutical companies, healthcare professionals, and patients. Understanding the essentials of clinical labeling is a commitment to patient safety and effective healthcare delivery.
As the pharmaceutical landscape continues to evolve, staying informed and proactive in addressing labeling challenges is paramount for success in bringing life-changing medications to the market. Partnering with a leader in clinical labeling services like Freyr will help you hasten your Regulatory approval process. Contact us today to learn more about our expertise.









