Introduction
In the pharmaceutical arena, the assurance of patient safety is of prime importance. Moreover, the ICH guidelines highlight patient safety by upholding a harmonized approach to be followed by countries/ regions. One such pivotal guideline is ‘ICH M7(R2) Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals To Limit Potential Carcinogenic Risk’ which is detailed in the blog ahead. Furthermore, the blog uncovers its implications for the pharmaceutical landscape, and glimpses into the future it heralds for drug safety.
Understanding DNA Reactive Impurities
DNA reactive impurities are akin to molecular whispers that hold the potential to interact with the very essence of life – DNA. In doing so, they might sow the seeds of mutations, which, over time, could escalate into the spectrum of carcinogenic risks. Identifying and controlling these impurities stand as the pillars of patient safety, guarding against unintended consequences that could ripple through lives.
Essence of ICH M7(R2) Guideline
ICH M7(R2) guideline is a comprehensive framework that guides the assessment and control of DNA reactive impurities in pharmaceuticals. This guidance is underpinned by the philosophy of science-based and risk-based approaches, translating scientific understanding into practical strategies that safeguard patient well-being.
Pillars to safeguard drug products from DNA reactive impurities:
1. Threshold of Toxicological Concern (TTC)
Within the complex weave of ICH M7(R2) guidelines, a revolutionary concept is – the Threshold of Toxicological Concern (TTC). This pragmatic notion posits that impurities existing at minute levels might not pose significant threats. The TTC principle reconciles stringent safety concerns with practicality, curtailing excessive testing without compromising the core tenet of patient safety.
2. Structural Alerts and Expert Judgment
ICH M7(R2) introduces a symphony of molecular notes in the form of structural alerts – motifs that hint at potential DNA reactivity. The guidance acknowledges the orchestra of data gaps that might arise and, in such instances, invites expert judgment to join the performance.
3. Tiered Approach to Assessment
ICH M7(R2) is a tiered approach to evaluate DNA reactive impurities. This ascendancy involves gauging factors such as exposure, potency, and structural analogies to known mutagens. By categorizing impurities systematically, pharmaceutical entities choreograph precise evaluation strategies.
4. Control Strategies and Risk Mitigation
ICH M7(R2) determines control strategies to combat potential risks that include steps from refining manufacturing processes to recalibrating specifications. By executing these maneuvers, pharmaceutical firms entities fortify the assurance that DNA reactive impurities remain dormant, far from the thresholds of concern.
Implications for the Pharmaceutical Industry
The resonance of ICH M7(R2) transcends the boundaries of a mere guideline; it reverberates through the corridors of drug development, manufacturing, and regulatory compliance. The following wheel chart details the implications in logical manner.
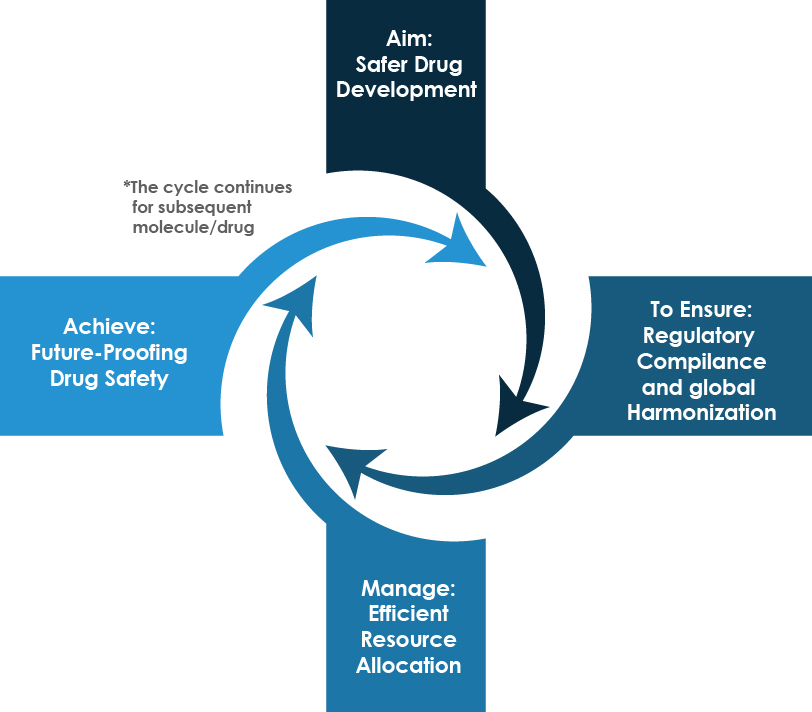
Upholding ICH M7 (R2) compliance, a continuous process
Role of Regulatory Vendors
As the pharmaceutical industry endeavors to adhere to the intricate notes of ICH M7(R2), regulatory vendors emerge as the maestros who orchestrate the harmony of compliance. These vendors are not mere service providers but envoys of expertise, guiding pharmaceutical firms through the symphony of safety. Their role is multifaceted:
1. Interpretation of Guidance: Regulatory vendors unravel the complexity of ICH M7(R2), offering insights into the nuances and implications. They translate the guidance into actionable strategies that resonate with each pharmaceutical firm's unique composition.
2. Expertise in Assessment: With a rich tapestry of experience, regulatory vendors assist in structuring tiered assessment approaches. They navigate the intricacies of structural alerts, expert judgment, and tiered evaluation, steering firms away from potential pitfalls.
3. Tailored Control Strategies: Regulatory vendors collaborate with pharmaceutical entities to sculpt tailored control strategies. They craft approaches that resonate with each firm's manufacturing processes, ensuring that control measures align harmoniously with operations.
4. Compliance and Documentation: The symphony of compliance demands meticulous documentation. Regulatory vendors compose the notes of documentation, ensuring that each action taken resonates with the melody of regulatory requirements.
Conclusion
The ICH M7(R2) guidance stands as a cornerstone in the citadel of pharmaceutical safety. It's a testament to the harmonious collaboration between global health regulatory bodies and the pharmaceutical industry, all with a singular aim – safeguarding patient welfare. By embracing the principles penned in this guidance, we illuminate the path toward a safer, more secure future for pharmaceuticals.
Regulatory vendors can streamline the pharma firm’s journey to better understand the regulatory technicalities and safeguard compliance until its conformity. Consult our medical writing expertise to explore more.