Overview
Regulatory experts across 120+ Countries, offering localized Regulatory services for Bio-Pharma, Medical Device, Consumer Healthcare & Generic companies
Freyr’s global pool of Regulatory experts with a deep understanding of regional/local Regulatory requirements of 120+ countries enable Life Sciences companies to expand to new markets, in an accelerated model. Catering to the worldwide Regulatory needs, Freyr’s global capabilities span across Local Regulatory Affairs, Health Authority Interaction, Regional Regulatory Intelligence, Regulatory/Medical Writing (including local language documents), End-to-end submission & publishing, artwork & labeling, IDMP, CMC, QA/Audit Services etc.- pertaining to Medical Devices, Pharmaceuticals/Drugs, Cosmetics, Biologics/Biosimilars and Food Supplements.
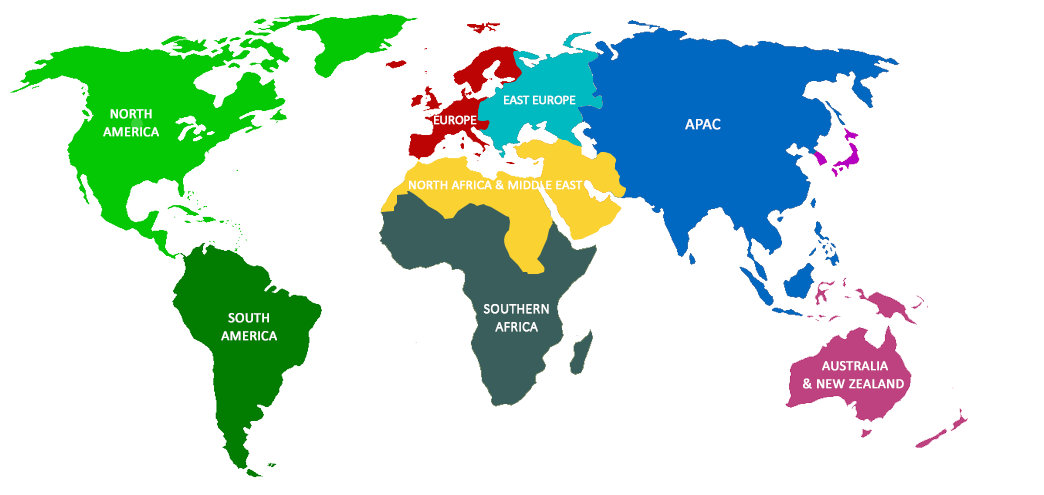
Freyr’s Global Presence
- Canada
- United States America (USA)
- Australia
- New Zealand
- Bangladesh
- Bhutan
- Brunei
- Cambodia
- China
- Dominican Republic
- Fiji
- Hong Kong
- India
- Indonesia
- Laos
- Malaysia
- Maldives
- Mongolia
- Myanmar
- Nepal
- Pakistan
- Philippines
- Singapore
- Sri Lanka
- Taiwan
- Thailand
- Timor Leste
- Vietnam
- Algeria
- Bahrain
- Benin
- Burkina Faso
- Burundi
- Cameroon
- Central African Republic
- Chad
- Djibouti
- Egypt
- Eritrea
- Ethiopia
- Gabon
- Gambia
- Ghana
- Guinea
- Guinea Bissau
- Iraq
- Israel
- Ivory Coast
- Jordan
- Kenya
- Kuwai
- Lebanon
- Liberia
- Libya
- Mali
- Mauritania
- Morocco
- Niger
- Nigeria
- Oman
- Palestine
- Qatar
- Rwanda
- Saudi
- Senegal
- Sierra Leone
- Syria
- Togo
- Tunisia
- Turkey
- United Arab Emirates (UAE)
- Uganda
- Yemen
- Albania
- Andorra
- Armenia
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Finland
- France
- Georgia
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Montenegro
- Netherlands
- Norway
- Panama
- Poland
- Portugal
- Romania
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- United Kingdom (UK)
- Kazakhstan
- Kosovo
- Kyrgyzstan
- Russia
- Serbia
- Tajikistan
- Turkmenistan
- Ukraine
- Uzbekistan
- Japan
- South Korea
- Argentina
- Bolivia
- Brazil
- Chile
- Colombia
- Costa Rica
- Ecuador
- French Guiana
- Guatemala
- Guyana
- Mexico
- Paraguay
- Peru
- Puerto Rico
- Suriname
- Uruguay
- Venezuela
- Angola
- Botswana
- Democratic Republic of the Congo
- Lesotho
- Madagascar
- Mauritius
- Malawi
- Mozambique
- Namibia
- Seychelles
- South Africa
- Swaziland
- Tanzania
- Zambia
- Zimbabwe