Overview
In the ever-evolving Regulatory landscape of Global Cosmetics Regulations, the Cosmetic Product Information File (PIF) stands as a cornerstone for Cosmetic product safety and Cosmetic Regulatory compliance in the ASEAN and European Union (EU) markets. A Product Information File for Cosmetics is not just a regulatory formality, it is a comprehensive dossier that validates product safety, efficacy, and quality.
At Freyr, we specialize in compiling, reviewing, and maintaining PIFs for Cosmetics, empowering brands to confidently access and thrive in highly regulated regions. Whether you're launching a new line or expanding your global footprint, our Cosmetic Regulatory experts ensure that your PIF meets all technical and jurisdictional requirements.

What is a Cosmetic Product Information File (PIF)?
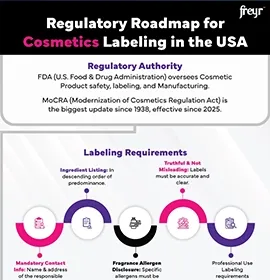
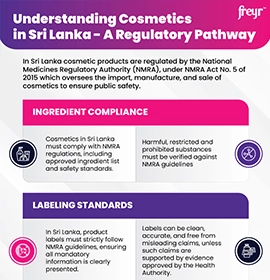
Under EU Cosmetic Regulation (Regulation (EC) No. 1223/2009) and ASEAN Cosmetic Directive, the Cosmetic PIF is a mandatory document required for each product placed on the market. It must be readily available to competent authorities for inspection and must remain updated throughout the product lifecycle.
The PIF Cosmetics dossier includes:

Description of the Cosmetic product

Cosmetic Product Safety Report (CPSR) – core of the Cosmetic Safety Assessment

Manufacturing method & GMP (Good Manufacturing Practices) compliance statement

Proof of the claimed effects

Complete Cosmetic product labeling details

Details of the Cosmetic Ingredient Review for substances used
How Can Freyr Help?
Freyr brings global experience and regional intelligence to the table. Our Cosmetic PIF services are tailored to suit local requirements while aligning with international best practices.
Why Choose Freyr?
As a trusted Global Regulatory Partner, Freyr offers a seamless and strategic approach to Cosmetic Product Registration and compliance:

Team of certified toxicologists, formulation scientists, and regulatory experts

Global reach with deep local expertise

Proven track record of 1000+ compliant cosmetic dossiers

Strong collaborations with Health Authorities (HAs) and testing laboratories worldwide

Cost-effective and scalable service delivery model
Unlock Market Access with Confidence
Whether you are navigating the EU cosmetic regulation, seeking ASEAN market entry, or preparing a Cosmetic Product Safety Report, Freyr ensures your brand stays compliant, credible, and competitive. Our structured and end-to-end approach helps simplify complex processes and accelerate cosmetic product registration across geographies.
FAQs – Cosmetic Product Information File (PIF)
1. What is a Cosmetic Product Information File (PIF)?
A Cosmetic Product Information File (PIF) is a mandated regulatory requirement for marketing cosmetic products in the EU and ASEAN regions. It’s maintained by the designated "Responsible Person" and must be available for inspection by regulatory authorities.
2. What information must be included in the PIF?
The PIF should include:
- A comprehensive product description
- A Cosmetic Product Safety Report (CPSR)
- Manufacturing process details and GMP compliance statement
- Evidence supporting the product’s claimed effects
- Label artwork (label mock-up or final version)
- Data on any animal testing performed
- Other miscellaneous details
3. Why is GMP compliance important?
Good Manufacturing Practices (GMP) ensure that cosmetics are consistently produced and controlled to quality standards. A statement affirming GMP adherence is required in the PIF to demonstrate product safety and regulatory compliance.
4. What is a CPSR and why is it essential?
The Cosmetic Product Safety Report (CPSR) is an analytical document divided into:
- Part A: Cosmetic product safety information
- Part B: Cosmetic product safety assessment
It’s crucial to prove that the product is safe for consumers under expected use conditions.
5. Who is responsible for preparing and maintaining the PIF?
The PIF must be held by the “Responsible Person” — typically the manufacturer or importer placing the product on the market. This person ensures the PIF is correctly compiled and available for at least 10 years after the last batch is sold.
6. Why might brands engage regulatory experts like Freyr for PIF preparation?
Given the technical complexity—such as compiling a CPSR, validating claims, ensuring GMP compliance, and aligning with regulatory frameworks—many companies partner with experts like Freyr. They can handle dossier preparation, updates, and compliance support across multiple markets.