Overview
Cosmetic product labeling is a critical aspect of marketing and regulatory compliance for Cosmetic products. Accurate and compliant labeling enables consumers to make informed choices and helps brands build trust in the marketplace.
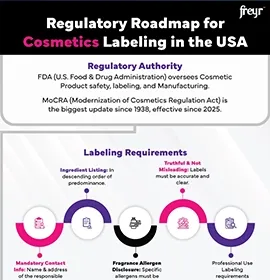
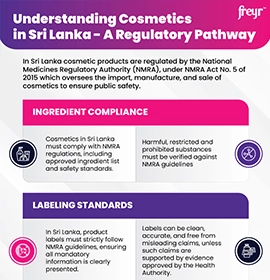
Cosmetic labeling requirements vary significantly across regions, encompassing essential details such as product identity, net content, directions for use, storage conditions, manufacturer/importer details, expiry information, ingredient lists, warnings, and mandatory language requirements. Navigating these region-specific Cosmetic labeling regulations—such as the EU Regulation 1223/2009, US FDA’s Food, Drug, & Cosmetic (FD&C) Act, Canada’s Food and Drugs Act, and the ASEAN Cosmetic Directive—is often complex, requiring meticulous review and expert guidance.
With Freyr’s Cosmetic product labeling review services, brands can confidently meet Cosmetic labeling compliance requirements across global markets, ensuring seamless approvals and quick market access.

Cosmetic Product Labeling Review – What is it?
A Cosmetic label review service involves assessing the artwork and content of Cosmetic product labels to ensure they adhere to the Cosmetic labeling requirements of the target market(s). This includes checking the correctness of:
Product identity
Net content
Directions for use
Storage information
Manufacturer/Distributor/Importer details
Expiry or best-before dates
Ingredient listing with verified INCI names
Region-specific warnings, cautions, and claims validation
Language and placement requirements
Given that global Cosmetic labeling regulations differ from region to region, products intended for multiple markets must meet each jurisdiction’s specific labeling standards. For instance:
EU Cosmetic labeling review ensures compliance with Regulation 1223/2009.
FDA Cosmetic labeling review ensures compliance with the FD&C Act and Fair Packaging & Labeling Act.
Canada and ASEAN also have unique packaging and Cosmetic ingredient compliance standards.
Freyr’s country-specific Cosmetic labeling services help brands navigate these complexities with ease.
How Can Freyr Help?
Why Choose Freyr?
Experienced team familiar with all Cosmetic categories, including skin care, hair care, baby care, oral care, and beauty products.
Proven expertise in reviewing 500+ Cosmetic labels across the EU, US, Dubai, Australia, ASEAN, India, and more.
Assistance in addressing region-specific regulatory complexities.
End-to-end Cosmetic regulatory consultation under one roof.
Affordable, scalable, and reliable Cosmetic label review sevice.
Adherence to tight timelines with hassle-free deliverables.
Structured approach that accelerates approvals and enables faster market entry.
Global partner network and strong ties with health authorities worldwide.
Partner with Freyr for robust Cosmetic product labeling review and achieve compliant, market-ready products effortlessly.
FAQs – Cosmetic Product Labeling Review
1. What is a Cosmetic product labeling review?
A Cosmetic product labeling review is a detailed assessment of your product’s label artwork and content to ensure it meets all Cosmetic labeling requirements in the target market. This includes checking INCI names, claims, mandatory warnings, net quantity declarations, and language compliance as per regional regulations.
2. Why is Cosmetic labeling compliance important?
Cosmetic labeling compliance ensures your product label is accurate, truthful, and adheres to the specific laws of each country. Non-compliant labels can lead to product recalls, fines, delayed launches.
3. Which regulations does Freyr’s Cosmetic label review service cover?
Freyr’s Cosmetic label review service covers major global regulations, including the EU Cosmetic labeling review under EU Regulation 1223/2009, the FDA Cosmetic labeling review under the FD&C Act, Canada’s Food and Drugs Act, and the ASEAN Cosmetic Directive. We also handle country-specific Cosmetic labeling for other markets worldwide.
4. What details are checked during a Cosmetic label regulatory compliance review?
Our team verifies product identity, net contents, directions for use, warnings, manufacturer/importer details, expiry date, storage conditions, ingredient list with proper INCI name verification, language requirements, and marketing claims against global Cosmetic labeling regulations.
5. How does Freyr validate Cosmetic claims?
We assess marketing claims on labels for truthfulness and regulatory acceptability. This Cosmetic claim validation helps ensure that no misleading or unsubstantiated statements are made, protecting your brand and ensuring Cosmetic regulatory compliance.
6. Can Freyr help with label artwork review as well?
Yes, our service includes a thorough label artwork review to ensure all mandatory information is clearly presented and meets layout, font size, and placement guidelines set by regional authorities.
7. Does Freyr support multi-market Cosmetic ingredient compliance?
Absolutely. Our team ensures Cosmetic ingredient compliance for each target market and confirms the correct INCI names appear on the label, avoiding ingredient-related non-compliance during inspections or audits.