Medical and Scientific Publication Services - Overview
‘Industry-focused’ publications are indispensable for Innovator Pharmaceutical, BioPharma, and Medical Devices companies to establish credibility, bridge research with clinical practice, educate HCPs to make informed decisions, obtain Regulatory approvals, and ultimately drive the market success of new therapies.
At the same time, complexities in managing fluctuating publication needs throughout the medicinal product and medical device lifecycle are inevitable, with publication workload peaks and troughs tied to various stages such as clinical trials, regulatory submissions, market launch, post-market surveillance, new indications, line extensions, or product enhancements.
Outsource your manuscript and publications to Freyr, a specialized, Industry-focused Medical and scientific publications vendor, to seamlessly navigate the publication complexities for various channels of dissemination of research findings, be it peer-reviewed journals, conferences, congresses, or symposiums.
Optimize your publication strategies, reduce your costs, accelerate your publication process, focus on your core research and product development activities, while we manage your high-quality publications and engaging impactful disseminations.
Manuscripts and Publications
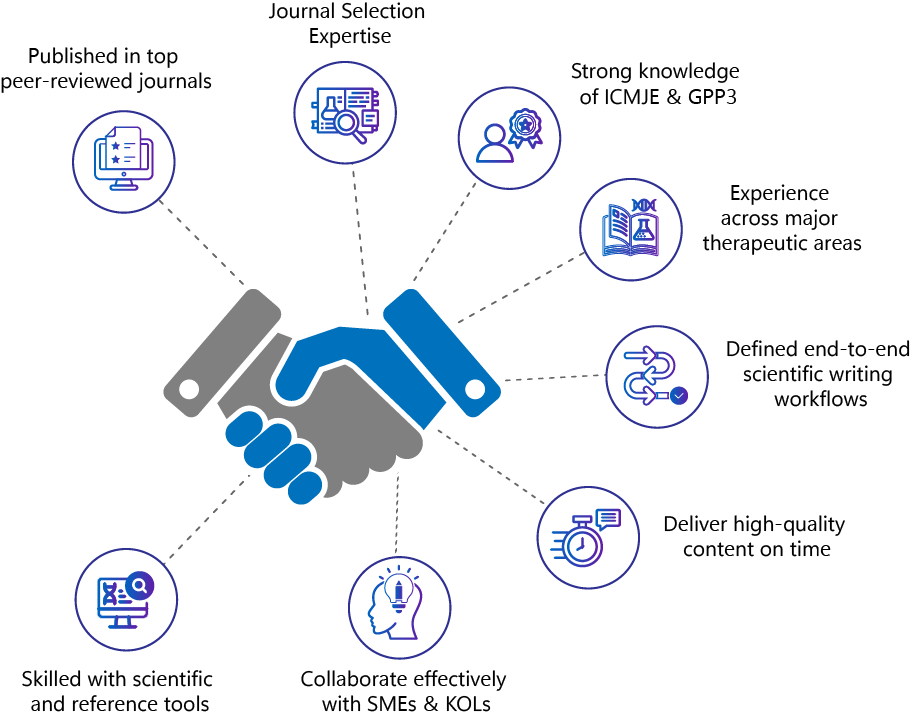
- Published multiple manuscripts in various peer-reviewed journals
- Shortlisting journals for publishing scientific content
- Knowledge of ICJME & GPP3 guidelines
- Vast experience in medical and scientific manuscript writing across multiple therapeutic areas like oncology, neurology, cardiovascular, psychiatry, respiratory, renal, gastrointestinal, etc.
- Defined processes for scientific writing through medical, editorial, and quality control reviews
- Experience working with stakeholders like SMEs, KOLs, and peer reviewers
- Timely delivery with the highest quality of scientific information & satisfaction
- Hands-on experience deploying the applicable tools, e.g., reference management, etc.