Remote and Virtual Audit Services - Overview
It is a known fact that drug manufacturers must work relentlessly on vaccine development and emergency product manufacturing to control health crises like COVID-19. In such scenarios, what if the manufacturers or regulators have no option for onsite audits? What options do they have to audit vendors located in different geographies across the globe? The only option is to move from a traditional audit to a remote audit process or a virtual audit. Unquestionably, remote audits/virtual audits are the future for conducting audits of low-risk processes that consist of documents, forms, and records that can be reviewed from a desktop.
Freyr’s expertise in remote and virtual audits services enables clients to choose the best-fit approach for their compliance needs. Our virtual audit capabilities are enhanced by secure technology and experienced auditors, making the transition from physical to digital seamless.
As a proven Regulatory partner for compliance practices, Freyr helps manufacturers comply with vendor audits. To ensure a smooth transition from onsite physical audits to remote/virtual audits, we offer remote audit services (virtual audit) and SOP review/writing services based on a unique 3-stage risk-based approach.
Our remote auditing methodology leverages advanced digital tools to conduct comprehensive, real-time assessments. This approach supports both remote audit and virtual audit requirements, providing flexibility and efficiency for global organizations.
Remote Audit - 3-Stage Risk-based Approach
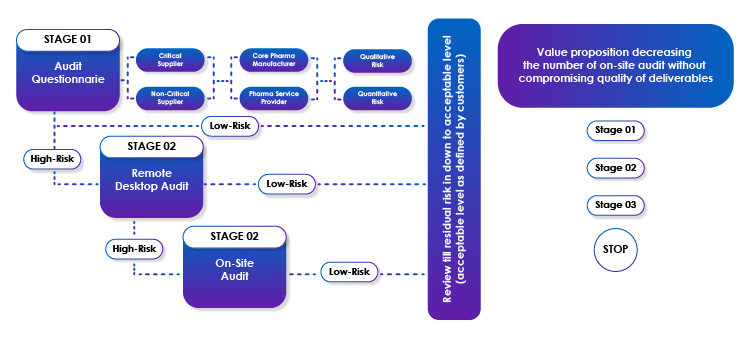
Audit - Stage-1
Freyr has existing audit questionnaires that can be further customized based on the criticality of the vendor, types of products/services offered by the customer’s vendor, type of risk analysis, etc. During this stage, we will liaise with the customer to customize the audit questionnaire and circulate the same to the customer’s vendors. Based on the response to the questions, the risk would be evaluated further, and it can be (mutually with the customer) decided whether the audit response is satisfactory, or should it require further remote desktop audit on selected agenda points.
Audit - Stage-2
Based on the risk analysis of the Stage-1 audit, Freyr will propose to perform a Stage-2 remote desktop audit, where required. This is not a re-audit and would include target areas where the risk was high as per the Stage-1 audit. The time and effort spent on Stage 2 is less than 30% of a full-time remote desktop audit.
Audit - Stage-3
Based on the outcome of the Stage-2 remote desktop audit, Freyr will recommend audit closure, or a further Stage-3 audit (an onsite audit). Based on the ability of the auditees to respond to Stage-1 and Stage-2 audits, a target audit agenda for on-site Stage-3 audits will be prepared with any pending items from Stage-1 and Stage-2 audits. The time and effort spent on Stage 2 is less than 10% of a full-time remote desktop audit.
This remote audit process is specifically structured to maximize efficiency and minimize disruption, making it ideal for organizations with global operations or travel restrictions.
The Freyr team is set to cater to all types of multi-site complex audit programs up to three hundred and sixty (360) virtual audits per year, with a combined experience of 144+ years.
Skillset and Global Footprint
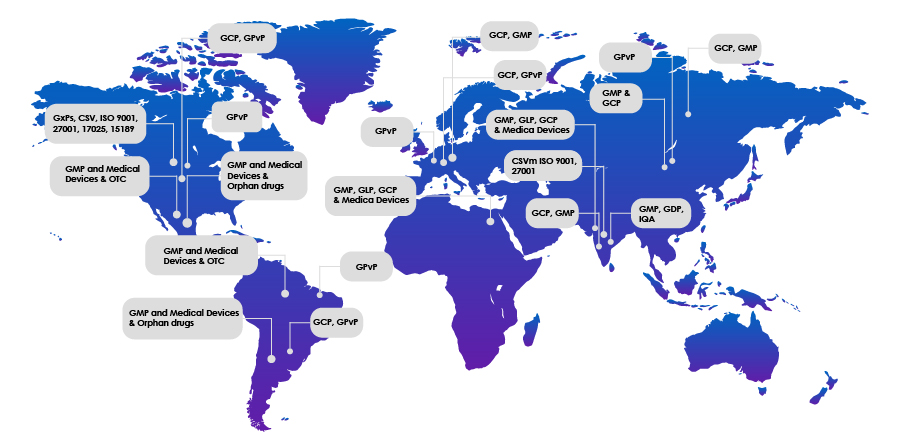
| GXP Auditor | Specialist in Remote Auditing | Geography | Countries to travel without Visa | Countries with prior audit experience |
|---|---|---|---|---|
| F1391 | GCP, GPvP, GMP, GAMP, CSV, ISO 9001, 27001, 17025, 15189, CCSK | USA/ India | US, UK, and Europe | USA, Canada, UK, Germany, Japan, and Australia |
| C_ARG_00122 | GPvP | LATAM | LATAM, EU, and USA | LATAM, EU, and USA |
| F1111 | CSV, ISO 9001 | India | 21 Visa Free Countries, 41 Countries Visa on Arrival | India |
| C_MEX_00596 | GMP and Medical Devices and Orphan drugs | Mexico (LATAM) | LATAM | LATAM |
| F1893 | GCP, GPvP, GMP | India | 21 Visa Free Countries, 41 Countries Visa on Arrival | India and LATAM |
| C_MEX_00222 | GMP, Medical Devices and OTC | Mexico (LATAM) | LATAM | LATAM |
| C_GBR_00774 | GPvP | UK | UK and US | LATAM, EU, and USA |
| F2042 | GMP, GDP, IQA | India | 21 Visa Free Countries, 41 Countries Visa on Arrival | India |
| C_IND_00948/1 | GMP and GCP | India | 21 Visa Free Countries | China |
| C_IND_00948/2 | GMP | India | 21 Visa Free Countries, 41 Countries Visa on Arrival | China |
| F2488 | CSV, GMP | India | 21 Visa Free Countries, 41 Countries Visa on Arrival | India |
| C_IND_00947 | GCP, GMP | India | EU and LATAM | China, Japan, Mongolia, EU, and LATAM |
| C_ISR_00949 | GMP, GLP, GCP and Medical Devices | Israel | EU and India | EU and India |
Remote and Virtual Audit Services
- Comprehensive remote audits
- State-of-the-art technology infrastructure (secured web-con/file-sharing/endpoint security policy etc.)
- Multi-site project management in a single audit plan/one iteration
- GMP, GP, GPvP, and GLP & QA expertise
- Experienced consultants to uncover the root causes of compliance issues, remediate them, and prevent recurrence right from toxicology to Pharmacovigilance
- Comprehensive audit coverage of twenty (20) process areas
- Accurate and on-time audit reports – “Factual” and “Findings”
- Integrated and compliant with multiple management systems (ISO 9001, 27001 with the USFDA, EMEA, and MHLW)

- Proven Regulatory partner for compliance practices
- Smooth transition to remote/virtual audits
- Remote audit & virtual audit services
- Unique 3-stage risk-based audit approach
- Extensive global experience and multi-site capabilities
- Comprehensive audit coverage across 20 processes
- Accurate, timely, and factual audit reports
