
In the pharmaceutical industry, ensuring patients have access to accurate and comprehensible information about their drugs is significant. The Core Patient Information Leaflet (Core PIL) is crucial in achieving this goal. It is a significant aspect of maintaining labeling compliance. In the following lines, we will provide an overview of what a core PIL is and the key elements involved in crafting a compliant one.
What is a Core PIL?
The Core PIL is a standardized document that accompanies prescription medicines. It is a legal requirement in many countries and is a vital communication tool between pharmaceutical companies and patients. The document includes details like the Summary of Product Characteristics (SmPC).
Enhance Your Core PIL Compliance Today
Request a Core PIL Consultation
Crafting a compliant Core PIL requires adherence to various Regulatory guidelines and standards. Listed below are the elements of a compliant Core PIL:
- Clarity and Readability: The language used in the document should be clear and easy to understand for the intended audience. Avoid medical jargon and complex terminology. Use simple language that can be easily comprehended by patients of varying literacy levels.
- Structure and Format: It should follow a standardized format to ensure consistency across different medications. It should include sections such as:
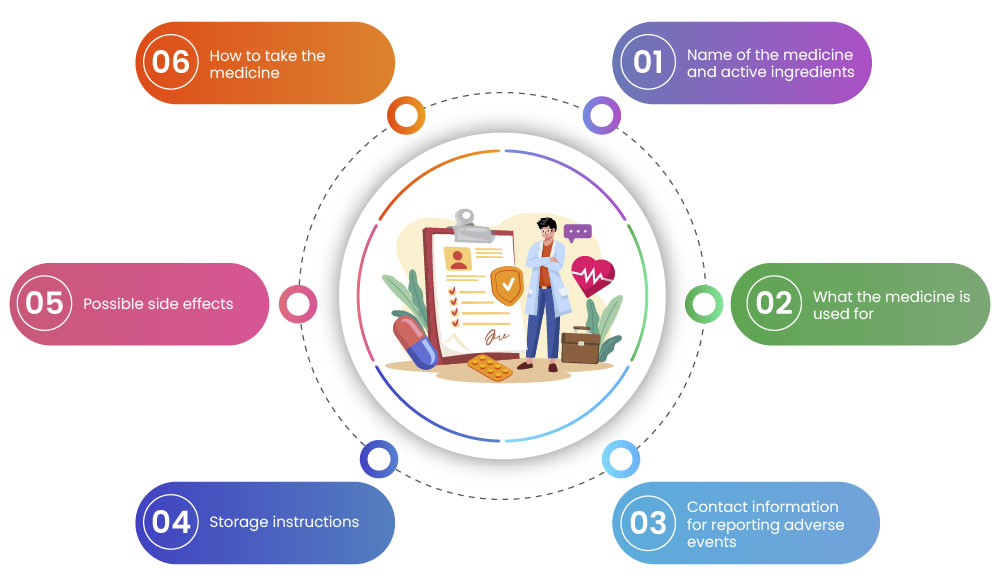
- Conciseness: While providing comprehensive information is essential, the Core PIL should also be concise and not overwhelm patients with excessive details. Focus on the most critical information that patients need to know.
- Visual Aids: Incorporating visual aids, such as diagrams or icons, can enhance understanding, especially for patients with limited language proficiency. Visuals can help illustrate how to take the medication or recognize warning signs.
- Translation and Localization: For multinational pharmaceutical companies, it is crucial to provide Core PILs in multiple languages to cater to diverse patient populations. Localization ensures that the content is culturally sensitive and relevant.
- Regulatory Compliance: Core PILs must comply with Regulatory requirements specific to each country or region where the medication is marketed. Ensure that the content aligns with the guidelines and regulations of the target market.
- User Testing: Before finalizing a Core PIL, consider conducting user testing with representative patient groups. This feedback can help identify any areas of confusion and refine the document accordingly.
Conclusion
Crafting a compliant Core PIL is a vital step in patient education and medication safety. By following Regulatory guidelines, structuring the document effectively, and using clear and concise language, healthcare providers can empower patients with the knowledge they need to make informed decisions about their healthcare.
As a part of labeling activities, it is important to remember that the Core PIL is a dynamic document that should be regularly reviewed and updated to reflect new information or changes in medication guidelines. By prioritizing patient education and employing labeling best practices, the Core PIL becomes a valuable resource for patients in their healthcare journey. Contact Freyr, an end-to-end Regulatory labeling expert, to create a compliant Core PIL.









